1. Introduction
The process of stabilization of oil and water droplets by solid particles has been acknowledged for over a century. Currently, these particle-stabilized emulsions are largely known as Pickering emulsions. They occur in personal care products, the food industry and have long been used in oil recovery and mineral processes (Bernardini 2015). These are industries where such emulsions are desirable for achieving certain characteristics of the products. In the oil and gas industry however, the formation of emulsion during oil production is a pricy problem, both in terms of chemicals used and the production lost (Kokal 2005). These emulsions have to be treated to remove the dispersed water and accompanying inorganic salts in order to meet market specifications, transportation requirement and to reduce corrosion and catalyst poisoning in downstream processing (Kokal and Wingrove 2000). Surfactants with small molecular weight or amphiphilic polymers have long been employed in certain industries in emulsion stabilization, either by reducing interfacial tension or forming a viscoelastic interfacial film. Although these surfactants have been well understood and are widely in use, they are not the only potential sources of stabilization of emulsions. Colloidal particles with suitable wettabilitypartially in both the dispersed and continuous phases can function as Pickering-type stabilizer by forming a physical barrier at droplet interface. This phenomenon is discussed in detail in section 6.2 of this review paper.
Several other researches have shown that asphaltenes are the prime stabilizers of water-in-oil emulsions and that resins are needed to solvate the asphaltenes (Fingas, Fieldhouse et al. 1999, Fingas and Fieldhouse 2003, Fingas and Fieldhouse 2004, Fingas, 2014a, Fingas, 2014b, Fingas, 2014c). Certain studies however found out that a synergy in stabilization occurs when asphaltenes and fine solids jointly stabilize an emulsion based on a certain fractional area ratio of 2:1 of asphaltenes to solids (Sztukowski and Yarranton 2005). In a similar work (Bobra, Fingas et al. 1992), established that waxes cannot act as emulsifiers by themselves, but can stabilize emulsions in combination with asphaltenes or resins. Thus, according to their findings, a concentration of 0.01 g/ml of asphaltenes did not produce a stable w/o emulsion, but when 0.05 g/ml of wax added, stable emulsions were formed. However, an asphaltene concentration of 0.03 g/ml without the presence of wax produced a stable emulsion. These findings were repeated by the authors of this paper, and the results found corroborated this result.
2. The emulsion problem
The problem of separating water from produced crude oil is as old as the oil industry itself. At the beginning of the oil industry, the water separation problem was handled by settling the free water from oil in open tanks or pits and the sludge (an intermediate phase between clean water and clean oil) was disposed of, ordinarily by burning (Meyer 1964). It was not more than a century ago that attention was drawn to the fact that “sludge” is an emulsion of crude oil and water, and that substantial amount of merchantable oil can be recovered from the emulsion (API 1961). By crude oil emulsions, we are referring to water-in-oil (W/O) emulsions because most emulsions are this type (Kokal and Wingrove 2000). Although oil-in-water (O/W) emulsions also form and are encountered in the industry, they are generally resolved in the same way W/O emulsions are resolved, except electrostatic treaters cannot be used on O/W emulsions (Kokal 2005). At the time when crude oil and water are leaving the wellbore of an oil well, tight w/o emulsions can form due to the turbulence in the choke valve at the wellhead (Janssen, Noïk et al. 2001). The formation of emulsion during crude oil production is a very costly operational problem. It occurs when hydrocarbon and formation water in the reservoir and in production pipes are extremely mixed under shear/turbulence, and in the presence of surface active agents (Fingas, Fieldhouse et al. 1999, Opawale and Osisanya 2013) (Ngai and Bon 2014).
The continuous phases of these emulsions depend on the water to oil ratio, the natural emulsifier systems contained in the oil, and the origin of the emulsion. The emulsifiers are complex chemically, and they come in different shapes and sizes. As new oil fields are developed and as production conditions change in older fields, there is a constant need for new, effective demulsification methods. The emulsion must be separated before the crude oil can be accepted for transportation, to meet the residual salt and water content quality criteria for a delivered crude oil. The water content must be less than 1% (Fink 2015).
2.1. Definitions of emulsions
Emulsions are thermodynamically unstable systems, since they will separate to reduce the interfacial area between the oil phase and the water phase, as a function of time (Sjoblom 2001). Emulsions are metastable systems typically formed in the presence of surfactant molecules, amphiphilic polymers or solid particles. The relative balance of the hydrophilic and lipophilic properties of these emulsifiers is known to be the most important parameter dictating the emulsion type: oil-in-water (O/W) emulsions are preferentially obtained with molecules which are rather hydrophilic whereas water-in-oil (W/O) emulsions are produced in the presence of hydrophobic molecules (Leal-Calderon and Schmitt 2008).
(Manning and Thompson 1991) defined emulsion as a quasi-stable suspension of fine drops of one liquid in another liquid (Roberts 1926). defined emulsion as a system containing two liquid phases, one of which is dispersed as globules in the other. Other researchers defined emulsion as a mixture of two mutually immiscible liquids, one of which is dispersed as very small droplets in the other, and is stabilized by an emulsifying agent (Aziz, Darwish et al. 2002) (Singh, Thomason et al. 2004) (Kokal 2008).
Another definition of oil field emulsions was proposed by (Roberts 1926). According to his work, he maintained that oilfield emulsions vary from extremely unstable types, which should more accurately be called suspensions, to extremely stable ones. Based on that, he classified emulsions into three classes, according to their behavior in the hand centrifuge. These are: (a) Emulsions that show only clear oil and; and are better referred to as suspensions, thus if allowed to stand will generally separate into their different phases without any form of treatment. However, certain unstable emulsions occur which are capable of resolution in the centrifuge, especially when diluted with gasoline, but which will not settle to oil and water without any form of treatment. (b) Emulsions that show the emulsion phase, with or without water, and a clear oil phase. These are real emulsions and must be treated to recover the emulsified oil (c) those that may or may not show emulsion and water phases, but which also show cloudy oil after centrifuging.
Something common to all the Definitions provided in this review and many others not stated here is the fact these emulsions are thermodynamically unstable and separate into two phases if allowed to sit for a long time (Singh, Thomason et al. 2004). These emulsions, which fall under macro-emulsions (having dispersed phase diameters greater than 0.1 μm) are said to be thermodynamically unstable systems because the contact between the oil and water molecules is unfavourable, and so they will always break down over time. There has been more comprehensive studies and a lot has been known about the formation and stabilization of oil-in-water emulsions than of the water-in-oil emulsions type (Oliveira and Goncalves 2005). To understand the W/O emulsions, more information is needed on the materials responsible for their formation and stabilization, and especially how solid particles form or enhance their stabilizations.
2.2. Classifications of emulsions
Decades of far-reaching research work on water-in-oil emulsions (often called “chocolate mousse”) that form after oil spill (Fingas and Fieldhouse 2009), found that four classes of emulsions form when crude oil mixes with water. Among the leading studies on classification of crude oil emulsion according to their stability are the works of (Fingas, Fieldhouse et al. 1999, Fingas and Fieldhouse 2003, Fingas, 2014a, Fingas, 2014b, Fingas, 2014c, Fingas and Fieldhouse 2014) who proposed new empirical data and corresponding physical knowledge of emulsion formation. Based on their studies, the density, viscosity, saturates, asphaltene, resins and fine solids were used to propose an emulsion type classification index which gives either an unstable, entrained, meso-stable or stable water-in-oil class of emulsion. From the four classes, only stable and meso-stable states can be considered as emulsions. It is assumed that the stability derives from the tough visco-elastic interface, triggered by asphaltenesand resins. Mesostable emulsions are the emulsions between stable and unstable emulsions. It is thought that meso-stable emulsions lack sufficient quantities of asphaltenes to render them completely stable. The meso-stable emulsions may degrade to form layers of stable emulsions. Given the oil and water phases, the type of emulsion formed depends on several factors. As a rule of thumb, when the volume fraction of one phase is very small compared with the other, the phase that has the smaller fraction is the dispersed phase and the other is the continuous phase. When the volume-phase ratio is close to 1 (a 50:50 ratio), then other factors determine the type of emulsion formed (McClements 2008) (Kokal and Wingrove 2000). Although emulsions are defined as stable dispersion of one liquid in another, not every mixture or dispersion of water in oil is an emulsion. For a dispersion to qualify as an emulsion, it has to be a stable dispersion (Bansbach 1965).
Based on this (Bansbach 1965), classified emulsions as Tight and Loose. Tight emulsions are those emulsion characterized by very small sizes of the dispersed phase, while a relatively larger dispersed phase droplet characterizes loose emulsions. According to (Bobra 1992) (Meyer 1964), the type of emulsifying agent determines the type of emulsion that would form, either w/o or o/w. If the emulsifying agent is more favorably wetted by the oil phase, the contact angle between the oil-water-solid boundaries, Ɵ, is greater than 90° and a w/o emulsion forms. However, if the water phase more favorably wets the particle, then Ɵ is < 90° and an o/w emulsion will form. If the contact angle is much greater or much lesser than 90°, the emulsion will be unstable. According to (Wang and Alvarado 2011), Stable emulsions form when the contact angle is near 90° As a rule of thumb, the continuous phase of the emulsion is normally the one in which the particles are preferentially dispersed.
(Fingas, Fieldhouse et al. 1999, Fingas and Fieldhouse 2003, Fingas, 2014a, Fingas, 2014b, Fingas, 2014c) classified emulsions into stable, mesostable, entrained and unstable. Each of these emulsion types has unique characteristics and is believed to be non-convertible to other types once formed.
(Kokal 2008, Fink 2015) in separate studies upheld that w/o emulsions form as a result of asphaltene and resin surfactant behaviors in oil of moderate viscosities (50–2000 mPa s) and that oil field emulsions are sometimes classified based on their degree of kinetic stability as Loose emulsions; those that will separate within a few minutes, medium emulsions; that will separate in approximately 10 min; and tight emulsions; that will separate within hours, days, or even weeks, and even then, not completely.
Earlier (Surfluh 1937), has classified emulsions as temporary and permanent. While a temporary emulsion will break down into oil and water by settling methods, a permanent emulsion would remain stable until it is treated effectively. Any method of preventing the formation of emulsions in oil and water mixtures must either reduce the degree of agitation or must employ the use of chemicals to produce physicochemical changes which will aid in emulsion prevention (Becker 1997).
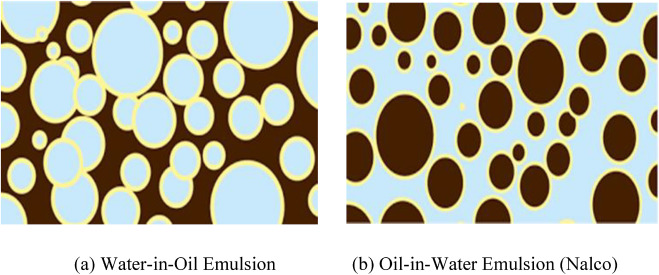
(Singh, Thomason et al. 2004) (Becker 1997) classified emulsions based on the size of the dispersed phase. When the dispersed droplets are larger than 0.1 μm, the emulsion is referred to as macro-emulsion. Emulsions of this kind are normally thermodynamically unstable (i.e., the two phases will separate over time because of a tendency for the emulsion to reduce its interfacial energy by coalescence and separation). Emulsions are called Micro-emulsions when the dispersed phase droplet sizes are less than 0.1 μm. Micro emulsions are transparent and can occur both as water-in-oil, or oil-in-water (as shown in Fig. 1).
 Fig. 1. (a) Water-in-oil emulsion (b) oil-in-water emulsion (Nalco).
Fig. 1. (a) Water-in-oil emulsion (b) oil-in-water emulsion (Nalco).Technically speaking, micro-emulsions differ from macro emulsions in several ways (McClements 2008) (McClements 2015), while there exist a direct oil-water contact at the interface of a macro emulsions, such direct contact is not present in micro emulsions. Also, macro emulsions are cloudy colloidal systems, while micro emulsions are optically transparent (isotropic).
2.3. Crude Oil Emulsions formed during Enhanced Oil Recovery (EOR) Processes
Enhanced Oil Recovery (EOR) technique has always been a subject of interest in the oil and gas industry. Prior to the fall in oil price which started June 2014, the high oil prices and energy demand all over the world has necessitated the needs for Enhanced Oil Recovery (EOR) methods. Of recent, EOR techniques are getting more attention by many countries since energy crises are getting worse and frightened. One of the reasons for this is due to the shortage of current oil resources and difficulties in finding new oil fields all over the world. EOR has been classified into five (5) categories, with general intent of reducing the mobility ratio between injected and in-situ fluids, eliminating or reducing interfacial tension or doing both simultaneously (Sheng 2014, Standnes and Skjevrak 2014, Talebian, Masoudi et al. 2014). Application of EOR technologygives an additional chance to get out more oil from the reservoir, possibly about another 20–40%. These classes are Mobility-control, chemical, miscible, thermal and other processes such as microbial. Despite the recorded successes, EOR processes are always accompanied with varying problems. The formation of strong emulsions and excessive formation of silicate scales (Umar and Saaid 2013) especially with the application of high concentration of alkali.
(Li, Lin et al. 2005) studied the effect of alkaline–surfactant–polymer (ASP) flooding using sodium hydroxide as the alkali constituent to enhance oil recovery of an onshore oilfield in Daqing, China. Although it has increased the oil recovery, it has also created a new problem for the industry. Although the crude oil is paraffinic (contains very little asphaltene), the alkali added formed stable w/o emulsion. The study reveals that the sodium hydroxide solution reacts with fatty acids in the aliphatic fraction of the crude oil and/or with the fatty acids formed from the slow oxidation of long chain hydrocarbons, and form soap like interfacially active components. These accumulate at the crude oil–water interface and contribute to the stability of the oil/water emulsion.
(Li, Xu et al. 2007) investigated the effects of HPAM on crude oil/water Interfacial properties and the stability of petroleum emulsions formed by Gudong crude oil. The investigation was conducted via measurement of interfacial shear viscosity, interfacial tension, Zeta potential, and emulsion stability. They found out that HPAM has the ability to adsorb at the interface between the oleic phase and water without decreasing the interfacial tension. Increasing the HPAM concentration however, leads to increase in the interfacial shear viscosity, Zeta potential, and stability of the emulsion.
(Abidin, Puspasari et al. 2012) in a comprehensive review of polymers used in EOR processes believe that there is an immense optimism that the use of polymer may play a significant role in resolving the current energy crisis since its applications in some EOR fields has shown some successes to recover more than 20% additional oil from OOIP. However, HPAM one of the most common polymers used in the EOR so far, was found to enhance the stability of o/w emulsions and makes the water treatment difficult (Li, Xu et al. 2007).
(Ahmadi and Shadizadeh 2012) investigated the implication of adsorption equilibrium when different types of nanosilica and Zyziphus Spina Christi, a novel surfactant, were combined in aqueous solutions for EOR and reservoir stimulation purposes. The study employed a conductivity technique to evaluate the adsorption of the surfactant and nanosilica in the aqueous phase. Batch experiments were used to understand the effect of adsorbent dose on sorptionefficiency as well. The results from this study can help in appropriate selection of surfactants in the design of EOR schemes and reservoir stimulation plans in carbonate reservoirs.
(Li, Lin et al. 2005, Li, Xu et al. 2007) at different times carried out laboratory studies concerning the chemical nature of the emulsions produced by Da Qing crude oil (paraffinic crude oil- that contains very little asphaltene and very low acid number). In this chemical flood where sodium hydroxide was used as the alkaline component in the recovery of crude oil, production was enhanced but the recovered oil was accompanied by a severely stabilized water-in-crude-oil emulsion. Certain studies however pointed to the fact that neither the surfactant nor polymer are responsible for the stabilization of the w/o emulsion.
In a series of articles, Ahmadi and co-workers (Ahmadi and Shadizadeh 2012, Ahmadi and Shadizadeh, 2013a, Ahmadi and Shadizadeh, 2013b, Ahmadi and Shadizadeh, 2013a, Ahmadi and Shadizadeh, 2013b, Ahmadi and Shadizadeh 2015, Ahmadi and Shadizadeh 2016) have conducted several studies ranging from estimation of adsorption behaviour of surfactants with nanosilica, adsorption of solid surfaces like carbonate reservoirs, adsorption of new plant derived surfactants on quartz among others. Results from these studies can help in making the right selection of surfactants in the design of chemical EOR schemes and reservoir stimulation plans in carbonate reservoirs. Also, the studies presented economically viable and environmentally friendly options for use in EOR techniques, particularly chemical flooding. Also, the studies are very helpful in understanding the mechanism of surfactant loss into sandstone reservoirs.
2.4. Some industry applications of emulsions- desirable emulsions
Despite the numerous challenges posed to the oil and gas industry by the formation of emulsions during crude oil production, emulsions and other materials like foams, have been used as mobility control or diverting agents in different EOR processes and many other useful applications (as shown in Table 1) in the oil and gas industry, food industry, construction industry, among others (Islam and Ali 1989, Israelachvili 1994).
Table 1. Some desirable and undesirable emulsions in the petroleum Industry (Schramm 2000).
| Occurrence | Usual Type |
|---|---|
| Undesirable Emulsions | |
|
Well-head Emulsions Fuel Oil Emulsions (Marine) Oil Flotation Process (Froth) emulsions Oil Sand Flotation Process (Diluted froth) Oil Spill Mousse Emulsions Tanker Bilge Emulsions |
W/O W/O W/O and O/W O/W/O W/O O/W |
| Desirable Emulsions | |
|
Heavy oil pipeline emulsions Oil Flotation Process froth emulsions Fuel-oil emulsion (70% heavy oil) Emulsion Drilling Fluid (Oil-Base Mud) Asphalt Emulsions Enhanced Oil Recovery in-situ Emulsions |
O/W O/W O/W W/O O/W O/W |
(Mendoza, Thomas et al. 1991) studied the effect of injection rate on emulsion flooding for a Canadian and a Venezuelan crude oil. The study was conducted using a porous media consisting of crushed Berea sandstone packed in 5 × 30 cm (diameter x length). The study employed a Lloydminster crude oil 16.4° API and 12° API Morichal crude oils. The brines used for the runs and for the preparations of the emulsions had sodium chloride concentrations of 3.3 and 7.5% by weight, respectively. The emulsions were prepared by adding 0.04% and 0.0004% by weight sodium hydroxide with pH = 12 and 10 respectively, to mixtures of crude oil and water. The study revealed that water driven emulsion flooding may offer a viable alternative to thermal recovery of moderately viscous oils.
(Abdul and Ali 2003) carried out a study to examine the effective techniques that can better water-flood bottom water reservoirs using polymer and emulsion as mobility control and/or blocking agents. In the provinces of Alberta and Saskatchewan certain light and moderately heavy oil reservoirs have a high-water saturation zone in connection with the oil zone. Using a conventional water-flood to produce from such reservoirs gives poor performance. This is attributed to insufficient and incomplete sweep of the reservoir by the injected water, which tend to move to the producing wells via those portions of the reservoir that have higher permeability. This leads to low recovery. In this study, polymer was used to control the movement of water in the oil zone while the emulsion was used to block the injected water from routing into the bottom water zone. The study found out that when producing from reservoirs with water leg, the use of 10% quality oil-in-water emulsion as a blocking agent and polymer solution as mobility control agent is the most successful strategy.
(Mandal, Samanta et al. 2010) investigated the efficiency of o/w emulsions as a displacement fluid during EOR process. In the study, they used synthetic emulsions prepared by gear oil, and experiments were conducted using sand pack flooding tests to observe the efficiency of the emulsion as displacing fluid. They found a substantial additional recovery (more than 20% of original oil in place) over conventional water flooding.
(Ashrafizadeh, Motaee et al. 2012) in a study of emulsification of heavy crude oil by surfactants reported several findings on the application of emulsions in the oil industry. He reported the work of (Kessick and Denis 1982) on pipeline transportation of heavy crude oil. According to them, conventional pipelining is not suitable for transporting heavy crudes from the reservoir to the refinery because of the high viscosities involved. This requires alternative transportation techniques (Saniere, Hénaut et al. 2004). in their study outlined several alternative transportation methods that have been proposed. Among the techniques proposed, transporting such viscous crudes as concentrated o/w emulsions is believed to be one of the most favorable ones (Poynter and Simon 1970, Marsden and Raghavan 1973, Sifferman 1981).
Water-in-diesel emulsions (WiDE) has been studied and applied as fuel for regular diesel engines for the reductions in the emissions of nitrogen oxides and particulate matters, which are both hazardous to our health, and reduction in fuel consumption due to better burning efficiency (Lif and Holmberg 2006). This leads to improvement in combustion efficiency when water is emulsified with diesel as a result of the micro-explosions, which assist atomization of the fuel. Several studies (Lin and Wang 2003, Abu-Zaid 2004, Lif and Holmberg 2006, Ghannam and Selim 2009, Alahmer, Yamin et al. 2010, Alahmer 2013, Fahd, Wenming et al. 2013, Ithnin, Noge et al. 2014) have been conducted on the viability of diesel emulsion as an alternative fuel. Most of the studies pointed to the fact that thermal efficiency is increased by using WiDE fuel compared to clean diesel fuel. Most of the studies also agree that WiDE result in improvements in brake power, torque and specific fuel consumptionmeasurements when the total amount of diesel fuel in the emulsion is compared with that of the neat diesel fuel.
3. The emulsification process
Crude oil emulsions form when oil and brine come into contact with each other, with the influence of sufficient mixing, and in the presence of an emulsifying agent or emulsifier. The amount of mixing and the presence of emulsifier are critical for the formation of an emulsion (Kokal and Wingrove 2000, Herrera 2012). Several sources of mixing are available during the process of crude oil production, a factor frequently referred to as the amount of shear. These include; Flow through reservoir rock, bottom-hole perforations/pump, flow through tubing; flow lines, and production headers, valves, fittings, and chokes, surface equipment, gas bubbles released because of phase change etcetera (Fingas 1995, Kokal and Wingrove 2000, Langevin, Poteau et al. 2004) as indicated with letters A to F on Fig. 2.
 Fig. 2. A schematic diagram of crude oil flow from the reservoir to the storage tanks.
Fig. 2. A schematic diagram of crude oil flow from the reservoir to the storage tanks.(Fingas, Fieldhouse et al. 1998, Langevin, Poteau et al. 2004, Fingas and Fieldhouse 2009) studied different emulsions and opined that the amount of mixing depends on several near-unavoidable factors. High speed agitation and shear causes vigorous mixing of oil and water and leads to smaller dispersed droplet sizes that are more stable. This is, however as a result of the increased energy transferred for the break-up process which eventually lead to small droplets and more stable emulsions. The sources responsible for this agitation may be present between the time at which the oil enters the well and the time when the produced phases are separated at the surface (Jackson, Harrington et al. 2012). Undoubtedly, certain methods of production contribute to the formation of emulsions.
Although, there seems to be no universal theory that has been postulated for all emulsions, several theories have been suggested to explain variations in emulsions formation processes (Lowe 1955).
According to (Clayton 1923, Becher 1988, Schramm 1992, Bhardwaj and Hartland 1994, Binks 2002, Fingas, 2014a, Fingas, 2014b, Fingas, 2014c, Umar, Saaid et al. 2017), many factors play different roles in the stabilization of emulsions, but the significance of such roles vary even as they combine in a single emulsion (Clayton 1923). presented various theories for emulsions. We would mention few of such theories here in section 3.1.
All crude oils have four main constituents belonging to four broad classes of compounds. These are classified as alkanes (also called saturates or aliphatics), aromatics, resins, and asphaltenes, SARA components. The lower-molecular-weight compounds in crude oils are generally alkanes and aromatics, while Asphaltenes, resins, and waxes (which are high-molecular-weight alkanes) account for the higher-molecular-weight compounds. In a complex mixture like petroleum, all these compounds interact in such a way that all components are maintained in the liquid oil phase. In other words, the lighter components of the oil act as solvents for the higher molecular-weight compounds. As long as this solvency interaction is maintained in the oil and thermodynamic conditions remain constant, the oil will remain stable. Should this equilibrium state be changed, a point will be reached where the solvency strength of the oil is insufficient to maintain the heavy components in solution, and as a result, they will precipitate out as solid particles. This is a frequent and problematic occurrence during petroleum production, transportation, and storage (Griffith and Siegmund 1985, Kawanaka, Leontaritis et al. 1989, Bobra 1991).
3.1. The phase-volume theory
This theory holds that, if small spheres of the same diameter are packed as closely as possible into a given space, they will occupy 74·048 per cent of the available volume, irrespective of the size of the spheres This fact was employed by Ostwald as the basis for a theory of emulsion, generally referred today as the “phase-volume theory.” According to Ostwald, 2 two types of emulsions are only possible over a certain range of concentration and that an emulsion of one liquid in another was only possible when the volume concentration of the dispersed liquid was less than 74 per cent, the double series being possible only over the range of 25·96 percent to 74·04 percent by volume.
3.2. The hydration theory of emulsions
According to this theory proposed by Fischer (Finkle, Draper et al. 1923), emulsions can only be created if the liquid which would form the continuous phase is all used in the formation of a hydrated compound of the emulsifying agent employed. Thus, substances such as acacia, soap, gelatin, casein, dextrin, and albumens are considered to act as emulsifiers in virtue of their ability to form colloidal hydrated compounds. The emulsifying efficiencies of these substances vary, since their “hydratability” varies qualitatively and quantitatively.
It is postulated by this theory that oil cannot be emulsified in a hydrated colloid until a certain minimum amount of water is present; correspondingly, too much water presence (exceeding the amount used in hydrating the colloid), makes the formation of stable emulsion impossible. It is reasonably accurate to emphasise the importance of hydrophilic colloids in forming oil-in-water emulsions, but it is only reasonable to extend this and debate that oil-soluble colloids (hydrophobic colloids) promote the formation of the water-in-oil type of emulsions.
3.3. Oriented wedge theory
This theory has been developed from the work of Langmuir and Harkins (Clayton 1923). It postulates the manner in which emulsions are stabilized. The theory is established upon the perception that the molecules of the emulsifier orientate themselves in the interface between the dispersed and continuous phases, forming a wedge, whose curvature determines the size of the dispersed phase.
3.4. The adsorbed film and interfacial tension theory
At present, the Interfacial tension theory is probably the most universally accepted theory of emulsions formations. Several works done by (Quincke 1889); in which he created emulsions from different oils in solutions of NaOH or gum Arabic. He found out that the interfacial tensions between the oils and these solutions were lower than those between the oils and pure water. Previous works (Langmuir 1917, Clayton 1923) had shown that oils containing free fatty acids result to better emulsions in dilute solutions of borax or sodium carbonate than those created by purer oils. (Quincke 1889), commenting on such works, recommended that the simplicity of emulsification differ with the acidity and viscosity of the oil, the concentration of the alkaline solution, and the solubility in water of the resulting soap (Clayton 1923). holds that with this theory “emphasis is laid upon the fact that emulsification is influenced by (1) the mass of the emulsifying agent present, (2) the ease with which this agent is adsorbed at the interfacial separating surface, and (3) the nature of the ions adsorbed by the resultant film."
4. Mechanism of emulsification
Emulsification is a process of agitating two or more immiscible liquids, which result in heterogeneous systems, consisting of at least one immiscible liquid intimately dispersed in another in the form of droplets, whose diameters, generally exceeds 0.1 μm (Baloch and Hameed 2005). The emulsification process comprises of a certain number of diverse chemical and physical processes and mechanisms, with many theories out forth to justify how differentemulsions are stabilized by the emulsifying agents. The emulsification history can begin right inside the reservoir where the crude oil and water comingle and squeezed through constricted pores. When the crude oil is produced from the well-head to the manifold (as shown in Fig. 2), there is usually a considerable pressure decrease with a pressure gradient over chokes and valves where the mixing of oil and water can be intense (Sjöblom, Aske et al. 2003).
As earlier discussed in the Definitions of emulsions, they are thermodynamically unstable material systems formed by at least two immiscible liquid phases, with one dispersed in the other(s). When such emulsions separate into their different phases, there is reduction in the free energy of the system as a result of the large decrease in interfacial area. However, the presence of a third component (referred to as a surfactant) in the erstwhile unstable system makes the spontaneous formation of thermodynamically stable dispersions (Shahidzadeh, Bonn et al. 1999, López-Montilla, Herrera-Morales et al. 2002). Two forms of emulsification processes are encountered and have been reported in the literature; (a) Spontaneous emulsification and (b) self-emulsification.
4.1. Spontaneous emulsification
On the one hand, also called “True” Spontaneous emulsification, and it ensues when two immiscible liquids are brought together, and they emulsify without the application of any form of external energy. The emulsification may last for few minutes, or several days depending on the nature of liquids involved (Shahidzadeh, Bonn et al. 1999).
4.2. Self-emulsification
On the other hand, emulsification in the industry is habitually accomplished with the aid of appropriate surface-active agents, and is commonly called 'self-emulsification', although the emulsification process is assisted by providing mechanical energy of some form, such as slight shaking, mixing (5) or sonication. In the case of self-emulsifying systems, the free energy required to form the emulsion is either very low and positive or actually negative (i.e., the formation is thermodynamically spontaneous) (Craig, Barker et al. 1995, Shahidzadeh, Bonn et al. 1999).
5. Conditions necessary for emulsion formation
All crude oils, whatever their origin contains certain characteristics which would likely make them emulsifiable (Bansbach 1965). For emulsions to form, three conditions must be satisfied (Smith and Arnold 1992). These conditions are (a) the two liquids forming the emulsion must be immiscible, (b) there must be sufficient agitation to disperse one liquid as droplets in the other, and (c) the presence of an emulsifying agent (Becher 1988, Bobra, Fingas et al. 1992, Smith and Arnold 1992, Fingas, 2014a, Fingas, 2014b, Fingas, 2014c).
According to Hany et al., (Aziz, Darwish et al. 2002), for an emulsion to form, the system must have the presence of water (brine), crude oil and sufficient agitation (Becker 1997). documented that the formation of emulsions requires; differences in solubility between the continuous phase and the dispersed phase, the existence of intermediate agents having partial solubility in both phases and the presence of an energy source or sources, sufficient enough to mix the phases.
5.1. The emulsifiers
Also, of paramount importance are the types of emulsifying agents or simply called emulsifiers. Emulsifiers are associated with the produced crude oil. Since the nature and compositions of crude oils vary so widely, there exists also a great variety of crude oil emulsifiers (Meyer 1964). To formulate concentrated stable emulsions, either oil-in-water or water-in-oil type, a third substance is required apart from the two liquids. This substance is called an emulsifying agent, or simply an emulsifier. The nature of the emulsifying agent determines what type of emulsion forms (Clayton 1923). Thus, the lastingness (known as stability) of the emulsion is dependent upon the rigorousness of the agitation and upon the emulsifying agents (Surfluh 1937).
(Roberts 1926) hold that the emulsifying agent responsible for the formation of petroleum emulsions is not categorically known. However, in a sizable number of cases, it is believed to be colloidal asphalt, which includes all asphalts and similar substances which occur in colloidal dispersion in crude oil. Since the nature and compositions of crude oils vary so widely, there exists also a great variety of crude oil emulsifying agents (Meyer 1964) . These emulsifiers include asphaltic materials, “resinous substances, soluble organic acids, particles in the ocean, particles found in crude oils including waxes and asphaltenes, particles found in sea water including suspended sediments, dissolved surfactants which accumulate at the water/oil interface including metallic salts, organic acids, organic bases and organometallics, and other tiny particles of solids, including products of corrosion of the equipment involved or particles of the producing formation, in case of wells completed in unconsolidated sands and sandy shales, are also the emulsifying agents contributing toward stability of the emulsions (Lee 1999).
The absence of these emulsifying agents in a crude oil can lead to the formation of a dispersion that will separate quickly due to rapid coalescence of the dispersed droplets. However, the presence of these emulsifying agents in the crude oil would lead to the formation of a very stable emulsion (Bobra 1992, Smith and Arnold 1992, Kokal and Wingrove 2000, Gafonova and Yarranton 2001, Janssen, Noïk et al. 2001, Sjoblom 2001, Binks 2002, Kokal 2002, Sjöblom, Aske et al. 2003, Fingas and Fieldhouse 2004, Sjoblom 2005, Müller and Weiss 2007). These natural emulsifiers form a mechanical film at the oil/water interface. The structural mechanical properties of the natural crude oil emulsifiers in the interfacial layer surrounding the dispersed droplets are believed are very important. This is the layer that provides resistance to coalescence in the final stage of emulsion breaking (Jones, Neustadter et al. 1978). It is worthy of mention here that, understanding the chemical and physical properties of these particles that reside at the interface is no doubt, key to understanding the emulsion breaking techniques. This area is receiving more attention of recent. The authors of this paper intend to expound more on the effects of native organic and inorganic solids on the stability of petroleum emulsions and how they can be included in emulsions stability prediction models.
(Gallup and Star 2004) in a study of Acidic crude oils identified that apart from their tendencies to cause scale formation in production tubing or in surface installations, acidic crude oils also have high tendencies of forming stable emulsions. The scale is often a mixture of calcium soaps associated with other minerals. Nigeria, on the Afia field, Indonesia, on the Attaka field, Great Britain, on the Blake field, Norway, on the Heidrun field, Angola, on the Kuito field, China, on the EDC field, Cameroon, on the Kita and Asoma fields. The acidity of crude by itself is not a sufficient criterion. Some weakly acidic oils in Cameroon or in Indonesia may form stable emulsions while other highly acidic crudes can be treated with no problem. It is the actual structure of the naphthenic acids that may explain these differences in behaviors, hence the importance of characterizing the naphthenic acids of a crude oil.
5.1.1. Amphiphiles
(Myers 1990) defined those substances that have chemical groups leading to surface activity as being amphiphilic (‘‘liking both’’), designating that they have some affinity for two fundamentally immiscible phases. The word amphiphilewas created by Paul Winsor 50 years ago (Paul and Moulik 1997). It emanates from two Greek roots. The prefix ‘amphi’ means “double”, “from both sides”, “around”, as in amphibian. Then the root philos which expresses affinity, as in “philanthropist” (the friend of man), “hydrophilic” (compatible with water), or “philosopher” (the friend of wisdom or science) (Salager 2002). Crude oil contains particles such as silica, clay, iron oxides; that are naturally hydrophilic, but can become oil-wet (hydrophobic) due to extended contact with the crude oil in the absence of water. A decrease in the size of oil-wet particles results in an increase in W/O emulsion stability. Emulsions with particles and asphaltenes combined can be much more stable than those stabilized by asphaltenes alone, provided that enough asphaltenes are present: all the adsorption sites on the particle surface need to be saturated by asphaltenes (Langevin, Poteau et al. 2004).
An amphiphilic substance exhibits a double affinity, which can be defined from the physico-chemical point of view as a polar-apolar division. On the one hand, an amphiphiles has a polar group made up of heteroatoms such as Oxygen (O), Sulphur (S), Phosphorus (P), or Nitrogen (N), incorporated in functional groups such as ether, alcohol, ester, thiol, acid, sulfate, sulfonate, phosphate, amine, amide etc. Equally, it has an principally apolar group which is a hydrocarbon chain of the alkyl or alkylbenzene type, sometimes with halogen atoms and even a few non-ionized oxygen atoms (Salager 2002).
Asphaltenes and resins are among the natural amphiphiles found in crude oils. In an attempt to determine the contribution of indigenous amphiphiles (the light, intermediate and the heavy ones) to emulsion stability, Dicharry et al.(Dicharry, Arla et al. 2006), evaluated and compared emulsion formed by different parts of substances. They found out that the emulsions formed with the light and intermediate fractions separated immediately when the agitation stopped. However, the most stable emulsions form with the fraction of crude that distilled at temperatures greater than 520 °C. This suggests that the amphiphiles with the highest molecular weight, i.e., resins and asphaltenes, play a major role in the protection of water droplet against coalescence, thus making the emulsion more stable. According to (Acevedo, Escobar et al. 1999, Yan, Elliott et al. 1999, Gu, Xu et al. 2002), the key role of the heaviest amphiphilic materials in the crude oil is to stabilize the interface, while the lightest ones tend to lower the emulsion stability. Due to its dual affinity, an amphiphilic molecule does not feel “at home” in any solvent, whether it is polar or non-polar. This is because, there always exist one of the groups which “does not like” the solvent environment. This is the reason amphiphilic molecules have a very strong tendency to migrate to interfaces or surfaces and to adjust so that the polar group lies in water and the non-polar group is placed out of it, and ultimately in oil. Amphiphiles have other properties other than tension lowering and this is why they are often categorized based on their main use such as: soap, foaming agent, detergent, emulsifier, wetting agent, dispersant, bactericide, corrosion inhibitor, antistatic agent, etc. In some cases they are known from the name of the structure they are able to build, i.e. membrane, micro-emulsion, liquid crystal, liposome, vesicle or gel (Salager 2002).
5.1.2. Asphaltenes, resins and waxes as emulsifiers
(Roberts 1926) in a study on treating field emulsions in Mid-continent Field that the emulsifying agent responsible for the formation of petroleum emulsions is not definitely known but, can be attributed to the presence of colloidal asphalt, that including all asphalts and similar substances which occur in colloidal dispersion in crude oil. Determining the type of an emulsion is very simple. If it is miscible with water, it is an emulsion of oil in water; if miscible with oil, it is water dispersed in oil. Probably more than 95 percent of all oil field emulsions are of the water in oil type (Wang, Zhang et al. 2004).
(McLean and Kilpatrick 1997) in an attempt to further investigate the effects of crude solvency and specific resin–asphaltene interactions on emulsion stability, created model emulsions, using model oil formed by dissolving varying amounts of resins and/or asphaltenes in a mixture of heptane and toluene. The resins and asphaltenes used in this study were isolated from four different types—Arab Berri, Arab Heavy, Alaska North Slope, and San Joaquin Valley. They found out that the prime factors governing the stability of these model emulsions were the aromaticity of the crude medium, the concentration of asphaltenes, and the availability of solvating resins in the oil (i.e., the resin/asphaltene or R/A ratio). The model emulsions were the most stable when the crude medium was 30–40% toluene and in many cases at small R/A ratios (i.e., R/A ≤1). This immensely supports the theory that asphaltenes are the most effective in stabilizing emulsions when they are near the point of incipient precipitation. The point of incipient precipitation, according to (Andersen and Speight 1992) is the point at which separation of asphaltenes from a crude oil becomes apparent.
(Sjöblom, Mingyuan et al. 1990b) in a study conducted to compare the destabilization of True petroleum Emulsions from the Norwegian Continental Shelf and Model Systems. It was reported that True water-in-crude oil emulsions are stabilized by a rigid interfacial film in which the surface-active material is accumulated, and that the distinct components of this film seem, at least for the crude oils from the Norwegian continental shelf, to be asphaltenes, waxes and other non-specified polar components. In addition, small wax particles are incorporated in the film. On the other hand, inorganic particlessuch as clays have not been detected under laboratory conditions.
Earlier before the works of Anderson and Speight (Bridie, Wanders et al. 1980), investigated the roles of wax and asphaltenes separately and in combination. In the study, a Kuwait 200 + fraction was first deasphaltenized (thirty-fold dilutions with n-pentane) and then dewaxed. The dewaxing involves a six-fold dilution in a methyl ethyl ketone/dichloromethane mixture 1/1 vol). The asphaltenes fraction was recovered and kept under nitrogen to prevent oxidation, and an emulsion of synthetic sea water (70% vol.) in the Kuwait 200 + fraction proper (30% vol.) was prepared. The investigation revealed that, the de-asphaltenized, dewaxed oil did not form a stable emulsion and had released 93% of its water content after standing for 15 min while the oil plus wax and asphaltenes mixed to the original concentrations gave a stable emulsion.
(Kim, Boudh-Hir et al. 1990) investigated the role of asphaltene both in wettability reversal and as a surfactant. The change in wettability is governed mainly by interfacial properties, with interfacial tension probably being the most important property. When a rock surface comes in contact with crude oil, the surface of the rock can possibly be modified due to asphaltene adsorption. This could alter the wettability of the rock. Whereas the polar segments of an asphaltene molecule are oriented towards the surface, the non-polar portions are away from it, causing the surface to be oil-wettable. It is a well-known fact that certain solids that possess dual wettability (i.e. are wetted by both oil and water can play the role of emulsifiers (Sjöblom, Söderlund et al. 1990a, Bobra 1991, Becker 1997, Lee 1999, Vignati, Piazza et al. 2003, Sztukowski and Yarranton 2005, Al-Sahhaf, Fahim et al. 2009)). Thus, when asphaltene adsorbs on such solids (when they come in contact with the crude oil), there is a high possibility of modification of its wettability from dual wettability to single wettability.
(Salager 1990) in an investigation of the most effective mechanism for destabilization of W/O or O/W emulsion reported that the removal of the surfactant from the water-oil interface by trapping it in a micro-emulsion is the most effective destabilization mechanism. According to the study, these emulsion stabilizers have a polar part with affinity to water and a nonpolar part with affinity to oil. These substances cannot fulfil this dual affinity, except when they are located at the water/oil interface, with the polar part immersed in water and the nonpolar part in oil. When they are adsorbed at the interface, they result to a decrease in the free energy of the system. Such include naphthenic acids, resins, asphaltenes, etc.
A physicochemical study by (Bobra 1991) on the emulsification of water-in-oil emulsions reiterated the fact that, the indigenous emulsifying agents are concentrated in the higher boiling fractions (boiling point > 370 °C), and predominantly in the residuum. It is largely accepted that asphaltenes (having hypothetical structures as shown in Fig. 3), resins, and waxes play key roles in emulsification, but specific mechanisms have not been clearly explained. These compounds are believed to be the main constituents of the interfacial films that surround the water droplets contained in the emulsion. These films have been shown to have high mechanical strength and therefore act as effective physical barriers, which prevent droplet coalescence and in turn gives rise to the stable petroleum emulsions.