1. Introduction
Polymers are essential materials in the modern society [1]. Over the past 60 years, polymers have transformed the agricultural production and food technology, improved health care through the introduction of functional medical devices, equipment, reduced the fuel consumption due to the fabrication of lighter vehicles, and enhanced the performance of aircraft. [1,2] All of these achievements were thanks to the synthesis of polymers in a diversity of properties, such as high mechanical performance and biodegradability [1]. The feedstocks used for the production of the polymers above mentioned are fuel/fossil-derivatives, primarily because they are easily assessable via petroleum and natural gas [1].
However, two main factors govern the gradual substitution of fossil-derived polymers by bio-based polymers [1,2]:
-
i.
The scarcity of feedstock and the rise in prices of petroleum and natural gas, which impose higher costs for polymers, and
-
ii.
The lack of environmental impact.
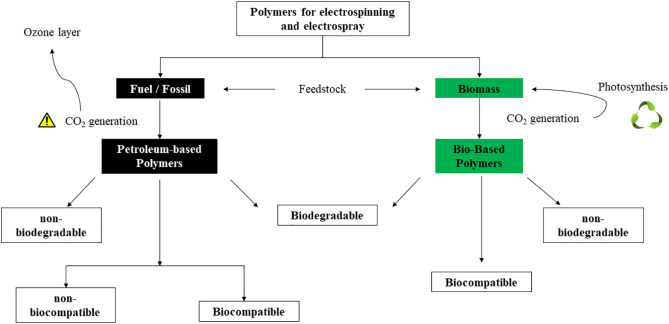
From The WWII to a few years ago, the petroleum-based plastics industry has powered the world's economy. Currently, the increasing demand for the petroleum-based raw material have been threatening the global economy and, most important, have been responsible for the world's climate change [1,3]. The release of carbon from petroleum-derived polymers by incineration or other degradation method increases the net greenhouse gasses in the atmosphere, which in turn, contributes to global warming (Fig. 1) [1,3].
 Fig. 1. Sources of polymers used in electrospinning/electrospray and its properties.
Fig. 1. Sources of polymers used in electrospinning/electrospray and its properties.Furthermore, it is important to highlight that fossil raw materials are a finite resource and, within a few generations, it will be depleted [1,3]. New resources, synthesis, and processing methods are strongly desirable to contribute to the reduction of the environmental impact generated by the current polymer industry [1].
A more sustainable level of development has to be reached, and one of the ways is to reduce the use of petroleum-based plastics [1,3,4]. Due to the advances in polymer science, the petroleum-based polymers can be replaced in nearly every function by bio-based polymers [1,3].
Bio-based polymers are materials derived from biomass (Fig. 1), according to the European Committee for Standardization (CEN) [5]. It means that bio-based polymers are mainly composed of non-fossil materials [6] and offer a renewable alternative to traditional petroleum-based polymers. The bio-based raw materials can be found in a variety of biological sources, like agricultural products (corn, potato, or soybeans), micro-organisms, algae, or crustaceans [1]. They can also be partially composed of renewable and synthesized polymers [1]. Some examples of commercialized bio-based polymers are:
-
•
Polylactides (PLA): derived from corn sugar, which is subsequently fermented to lactic acid and, finally, polymerized into poly(lactic acid) [2,7];
-
•
Polyhydroxyalkanoates (PHAs/PHBs): a plant-derived intercellular sugar or lipid is fermented by micro-organisms, giving rise to linear polyesters [2,8];
-
•
Polyols: sugar-alcohols-derived polymers. The most used monomeric polyols are glycerin and ethylene glycol. They have been commercialized and used by urethane industries [2,9].
-
•
Polyamides: Nylon-11 (poly (undecanoic amino acid)) is a polymer derived from castor oil [2,10].
-
•
Bio-PET: the bio-poly (ethylene terephthalate) is a combination of bio-based ethylene and other petroleum-based feedstocks. The ethylene portion is obtained by corn fermentation and is subsequently synthesized in the same process as a traditional PET [2,11].
-
•
Butyl rubber: a renewable version of synthetic butyl rubber that is a copolymer of isobutylene and BioIsoprene™. The genetically modified E. coliis used to produce the enzyme isoprene synthase, which polymerizes a sugar solution, giving rise to BioIsoprene™ [2,12].
-
•
Cellulose acetate: obtained from cellulose present in crops and leaves of plants [13].
Natural polymers are macromolecules that can be found in nature. Among them are proteins (e.g., collagen, gelatin, hyaluronic acid, zein, silk), polysaccharides(e.g., starch, cellulose, alginate, chitosan), terpenes (e.g., natural rubber), and lipids [[14], [15], [16]]. The advantage of using natural polymers is their ability to mimic the chemical environment of nature, primarily by giving biocompatibility. In contrast, they present weak mechanical properties, resulting in fragile materials in comparison to synthetic polymers [14,16].
In the last decade, more attention has been given to the use of bio-based and natural polymers as biomaterials for tissue engineering, wound dressing, drug delivery systems, and smart alternatives for biotechnology. It is worth to mention that using a polymer from a renewable source for any application in food, pharmaceutical, or medicine moves towards a more sustainable, affordable, and less dependent technology. Regardless of the area of application, the use of the expression “natural polymers” and electrospinning has constantly been growing in the last decade, unlike the concept of “bio-based polymers” to describe synthetic materials of natural origin, as demonstrated in Fig. 2. We can observe growth in the number of publications per year concerning to electrospinning of natural polymers, while the electrospinning of bio-based polymers remains very low. This result can highlight an urgent need for the development of new polymer molecules from renewable sources which present properties like biodegradability, biocompatibility, superior spinnability, and mechanical properties to be used in electrospinning/electrospray technology.
 Fig. 2. Schematic representation of the number of publications per year using the expressions “natural polymers” and “bio-based polymers” over the last 10 years following Web of Science.
Fig. 2. Schematic representation of the number of publications per year using the expressions “natural polymers” and “bio-based polymers” over the last 10 years following Web of Science.Given the importance of this issue, the ASTM's Subcommittee D20.96 on Biobased Products and Environmentally Degradable Plastics has published the “ASTM Biobased Content Briefing Paper,” which aims to standardize the application and commercialization of bio-based polymers and help with definitions and examples for determining bio-based content [17] (Fig. 3).
 Fig. 3. Schematic overview of main applications in electrospinning and electrospray of bio-based and natural polymers.
Fig. 3. Schematic overview of main applications in electrospinning and electrospray of bio-based and natural polymers.2. Electrospinning and electrospray processes
2.1. Electrospinning
Polymeric fiber mats produced by electrospinning have been in the spotlight of materials research and development for more than a decade [[18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]]. Although fiber mats production can be achieved through different techniques (i.e., design, phase separation, self-assembly, and template synthesis), the electrospinning generates a remarkable interest in the biomaterials field. It presents properties that usually cannot be found by using standard technology, as its capacity of production and scale-up, high porosity and microporosity, large surface area, easy to change topography, and interesting mechanical properties [18,24,29].
These characteristics make the electrospinning an important technique regarding the biomaterials field especially by the fact that fiber mats can present similar topography to biological systems, what can enhance and improve its efficiency in mimicking biological signs [[30], [31], [32]]. Furthermore, the increased surface area of nanofiber mats provides high availability of atoms on its surface giving rise to new physical and chemical properties that are assigned to the material at the nanoscale [33]. Some of the main features of electrospun/electrospray bio-based and natural polymers are summarized in Table 1.
Table 1. The most commonly used natural and bio-based polymers to produce fiber mats and microparticles through electrospinning/electrospray technique, and their main features.
| Polymer type | Polymer | Source | Main material's properties | Electrospinning/electrospray features | Ref. |
|---|---|---|---|---|---|
| Natural | Alginate | Brown seaweed | Hydrogel-forming. | The high viscosity of alginate solutions along the jet axis under DC field can produce uniformly dispersed microspheres/microcapsules through electrospray. | [[34], [35], [36]] |
| Cellulose | Plant fibers and wood | Film-forming, with high tensile strength. The high rate of water absorption. | Depending on the solvent used for electrospinning cellulose, the polymer can change its crystallinity, what eventually influences its spinability. Electrospun cellulose can give rise to smooth nanofibers with excellent mechanical properties: high tensile strength and flexibility. | [37,38] | |
| Chitosan | Shells of crustaceans | Film and hydrogel-forming. It shows antimicrobial properties. | Electrospun chitosan can be obtained from highly acidic solutions. The degree of acetylation of chitosan plays a fundamental role in the electrospinning process. Antimicrobial properties can be tuned due to the high area per volume ratio of nanofibers. | [[39], [40], [41]] | |
| Gelatin | Hydrolysis of collagen from skin, bone, and connective tissues of animals | Hydrogel and film-forming. Highly soluble in water. It has an affinity for cells and other biomolecules. | The applied voltage during electrospinning has a substantial impact on fiber's diameter and morphology. The most prominent property of gelatin nanofibers is the ability to mimic the structural ECM microenvironment. | [[42], [43], [44]] | |
| Pullulan | Fungal exopolysaccharide (Aureobasidium pullulans) | Film-forming. | Its high extensional viscosity can efficiently produce nano- and microfibers under DC field and extensional strength. | [45,46] | |
| Zein | Corn | Film-forming. Unlike most natural polymers, zein is hydrophobic and non-water soluble, eliminating the need of crosslinking steps in a final material. | Electrospun zein can be obtained as nanofibers or nanoribbons. It can produce water-resistant nanofibers from ethanolic solutions. | [47,48] | |
| Biobased | Polyamide-6 (PA-6) | Ring-opening polymerization of caprolactam | Excellent mechanical properties. Can be processed through different methods. Chemically similar to collagen. | Nanofibers or ultrathin nanofibers can be obtained by electrospinning PA-6 acidic solutions. The PA-6 nanofibers can resemble collagen structure and have been highly effective as scaffolds for bone regeneration. | [49,50] |
| Polycaprolactone (PCL) | Ring-opening polymerization of ε-caprolactone or condensation of 6-hydroxy caproic acid | Film-forming. A wide range of processability. Peculiar mechanical properties which confer high tensile strength and viscoelasticity to materials. | PCL nanofibers morphology is very sensitive to the electrospinning process parameters. It can show different pores sizes depending on the fiber density in the unit area. | [51,52] | |
| Polylactic acid (PLA) | Ring-opening polymerization of lactide or direct condensation of lactic acid monomers | Film-forming. Its mechanical properties can vary from soft and elastic to stiff and high strength materials, depending on the polymer Mw. Can be processed through different technologies. Degradation occurs by hydrolysis and can take from months to years. | Solvent type and PLA concentration can change the fibers morphology. This property can be used for different applications, from scaffolds for cell culture to enzyme immobilization and water filtration. | [7,53,54] | |
| Poly (lactic-co-glycolic acid) (PLGA) | Polycondensation of glycolic acid and lactic acid or ring-opening polymerization of lactide and glycolide | Its physical properties depend on the Mw of monomers, exposure to water, and storage temperature. Degradation occurs by hydrolysis and can take from days to months. | PLGA nanofibers can provide the suitable chemical and mechanical properties for cells and tissue growth, from cardiomyocytes culture to bone regeneration, while resembling the natural ECM microenvironment. | [55,56] | |
| Polyvinyl alcohol (PVA) | Polymerization of vinyl acetate followed by transformation to polyvinyl alcohol | Film- and hydrogel-forming. Water soluble, what requires crosslinking the final material. | Electrospun PVA nanofibers show good mechanical strength. However, they are very hydrophilic. They are an excellent alternative as scaffolds for cell culture or wound dressing materials due to their high affinity to biomolecules. | [57,58] |
Electrospinning can produce micro- and nanofibers from polymeric solutions or melt polymers [24,30]. The electrospinning apparatus is commonly represented by a syringe with a needle attached to the syringe tip, which is directed to a metallic base that acts as a support for the fiber mat collection. Both the needle and the metallic base are connected to a high voltage power source through electrodes (Fig. 4).
 Fig. 4. Illustration of the electrospinning process: A) electrospinning equipment, consisting of a high voltage source, syringe, and collector plate, B) gelatin fiber mat photo – macroscopic view (from the author's personal archive), C) optical microscopy image of gelatin nanofibers [59] (reprinted from Innovative Food Science & Emerging Technologies, Vol. 20, M. Nieuwland, P. Geerdink, P. Brier, P. van den Eijnden, J. T.M.M. Henket, M. L.P. Langelaan, N. Stroeks, H. C. van Deventer, A. H. Martin, Food-grade electrospinning of proteins, pp. 269–275., Copyright (2017), with permission from Elsevier), D) SEM image of PU/cellulose acetate/zein [60] (reprinted from Carbohydrate Polymers, Vol. 102, A. R. Unnithan, G. Gnanasekaran, Y. Sathishkumar, Y. S. Lee, C. S. Kim, Electrospun antibacterial polyurethane–cellulose acetate–zein composite mats for wound dressing, pp. 884–892, Copyright (2017), with permission from Elsevier), and E) TEM image of gelatin/chitosan core/shell nanofibers [61] (reprinted from Carbohydrate Polymers, Vol. 136, K. Jalaja, D. Naskar, S. C. Kundu, N. R. James, Potential of electrospun core-shell structured gelatin–chitosan nanofibers for biomedical applications, pp.1098–1107, Copyright (2017), with permission from Elsevier).
Fig. 4. Illustration of the electrospinning process: A) electrospinning equipment, consisting of a high voltage source, syringe, and collector plate, B) gelatin fiber mat photo – macroscopic view (from the author's personal archive), C) optical microscopy image of gelatin nanofibers [59] (reprinted from Innovative Food Science & Emerging Technologies, Vol. 20, M. Nieuwland, P. Geerdink, P. Brier, P. van den Eijnden, J. T.M.M. Henket, M. L.P. Langelaan, N. Stroeks, H. C. van Deventer, A. H. Martin, Food-grade electrospinning of proteins, pp. 269–275., Copyright (2017), with permission from Elsevier), D) SEM image of PU/cellulose acetate/zein [60] (reprinted from Carbohydrate Polymers, Vol. 102, A. R. Unnithan, G. Gnanasekaran, Y. Sathishkumar, Y. S. Lee, C. S. Kim, Electrospun antibacterial polyurethane–cellulose acetate–zein composite mats for wound dressing, pp. 884–892, Copyright (2017), with permission from Elsevier), and E) TEM image of gelatin/chitosan core/shell nanofibers [61] (reprinted from Carbohydrate Polymers, Vol. 136, K. Jalaja, D. Naskar, S. C. Kundu, N. R. James, Potential of electrospun core-shell structured gelatin–chitosan nanofibers for biomedical applications, pp.1098–1107, Copyright (2017), with permission from Elsevier).The syringe stores the polymer (melt or in solution) and when the electrostatic forces overcome the surface tension of the liquid, the fibers start to be formed. At this time, the surface of the fibers undergoes repulsion of the free charges; at the same time, the elasticity of the fluid produces an unstable drop in the shape of a cone, which is formed at the tip of the needle (Taylor cone). Then, an electrically charged thin fluid jet is released from the Taylor cone to the collector base that is oppositely charged. This liquid filament formed is accelerated and stretched and undergoes deformations or instabilities associated with the fluid, to the electric field, or to the aerodynamics of the jet until its arrival at the metal collector, ending up in a fiber network or three-dimensional matrix [24,62].
Aside from the high surface/volume ratio, porosity control and the interconnected pores system, fibers orientation, morphology, and sizes, the polymer composition (blends and composites) can give rise to specific properties according to the desired application [28,30,63,64].
Electrospun fiber mats show high porosity and a film morphology that is suitable for a post-processing surface modification, what can open a range of possibilities for bio-functionalization, for example.
2.2. Electrospray
In electrospray, micro and nanoparticles (spheres or capsules) can be obtained from a polymer in solution with a conductive solvent. The technique is based on similar principles of the electrospinning process. However, the difference between the two techniques consists of changing the properties of the solution, like concentration, solvent, and viscosity, and the process parameters as flow rate, distance from the tip of the needle to the collector, and voltage. Also, during the jet formation from the Taylor cone in electrospray, the changes in the parameters can result in the break of the jet into droplets giving rise to particles of different sizes and shapes (Fig. 5) [23,[65], [66], [67]].
 Fig. 5. A representative illustration of the electrospray process. A) electrospray equipment, like electrospinning. A bath collector is usually used for the production of microparticles; B) SEM image of electrospray casein/PBS microparticles [68] (reprinted from Food Hydrocolloids, Vol. 28/Issue 1, A. López-Rubio, E. Sanchez, S. Wilkanowicz, Y. Sanz, J. M. Lagaron, Electrospinning as a useful technique for the encapsulation of living bifidobacteria in food hydrocolloids, pp.159–167, Copyright (2017), with permission from Elsevier).
Fig. 5. A representative illustration of the electrospray process. A) electrospray equipment, like electrospinning. A bath collector is usually used for the production of microparticles; B) SEM image of electrospray casein/PBS microparticles [68] (reprinted from Food Hydrocolloids, Vol. 28/Issue 1, A. López-Rubio, E. Sanchez, S. Wilkanowicz, Y. Sanz, J. M. Lagaron, Electrospinning as a useful technique for the encapsulation of living bifidobacteria in food hydrocolloids, pp.159–167, Copyright (2017), with permission from Elsevier).Micro- and nanoparticles obtained by electrospray can show higher loading efficiency and narrow particle size distribution compared to particles obtained by other techniques [66]. Furthermore, the need for particle separation from dispersing solution or even nondegradable surfactants employed in some of the fabrication techniques might cause further difficulties. By electrospraying, the particle synthesis can be obtained by a single step processing, and there is no need of surfactant [65,66,69].
3. Current applications of electrospinning/electrospray of bio-based and natural polymers
3.1. Food technology and food packaging
The use of electrospinning and electrospray-based materials have expanded in the food industry as its use can be found in nutrition, food preservation, and packaging fields [70]. In food technology and packaging applications, general requirements are mandatory before processing polymers, i.e., polymers should be biodegradable, non-toxic, and food grade, which must be soluble in non-toxic solvents [71,72]. Most of the biopolymers used for electrospinning and electrospray in the food industry are derived from renewable sources like alginate, chitosan, starch, and gums [70,71].
Regulatory aspects concerning nano-based food additives and food packaging are still to come or under review as the technology advances, and there are different approaches and points of view related to the commercialization of food nanotechnology around the world [70,71]. While in the European Union, there are legislative provisions that regulate and control aspects like risk assessment of nano-based food ingestion and food contact, the Food and Drug Administration (FDA) in the USA does not have any specification nor regulation for nano-based food products. Instead, they do require a pre-market authorization for some substances, and there is no need for this requirement when the ingredients are classified as “generally recognized as safe” (GRAS) [71].
3.1.1. Food additives
Electrospray particles and electrospun fibers have been widely explored for encapsulation of as antioxidants. In particular, zein and gelatin have demonstrated excellent encapsulation profiles and stabilities either by containing β-carotene [73] or (−)-epigallocatechin gallate (EGCG) [74]. In a different approach, Nieuwland and co-workers [59] demonstrated the feasibility of globular proteins as food additives by electrospinning them with gelatin to create building blocks for texturally meat replacers.
Another exciting application of electrospray particles as food additives is the encapsulation of probiotics. López-Rubio and co-workers [68] encapsulated Bifidobacterium strains into whey protein/pullulan sub-micron capsules whose average diameters ranged from about 650 to 260 nm. These nanoparticles were able to prolong the probiotic storage at 4 °C and 20 °C in an environment with high relative humidity. Recently, Coghetto and co-workers [35] also demonstrated that the microencapsulation was able to improve the storage stability of Lactobacillus plantarum BL011 by incorporating the probiotic into alginate/pectin electrospray microparticles. This system was able to prolong the survival of the probiotic cells at 4 °C for 21 days [35].
In a different approach, Fung and co-workers [75] successfully obtained agro waste nanofibers (solid waste of soybean, oil palm trunk, and oil palm frond) containing Lactobacillus acidophilus with an encapsulation yield of 78.6%–90% of survival probiotic, that also demonstrated excellent cell viability results during 21 days of storage under refrigeration.
3.1.2. Antimicrobial packaging
Active antimicrobial food packaging shows the advantages of passively protect food against environmental factors and microbial growth. However, the definition “active” means some performance other than providing an inert barrier to external conditions [72,76]. Active packaging is employed as a strategy to release antimicrobial agents in a sustained way to the food surface. This packaging acts by inhibiting or retarding microbial growth and, consequently, extending food shelf life [76].
The incorporation of eugenol/cyclodextrin (CD) complex inclusion in poly (vinyl alcohol) (PVA) nanofibers improved the thermal stability of eugenol [77]. Although its germicide and fungicide properties, eugenol is an easily oxidized label molecule that can be decomposed when exposed to oxygen, light, or heat. In addition to the thermal stability, the eugenol/CD-PVA nanofibers presented a slower release of eugenol, what makes of this association a potential active food packaging [77].
Chitosan has also been explored in food packaging due to its bactericide property [78,79]. In this sense, Wang and co-workers developed a PVA nanofiber mat containing chitosan/nano-ZnO composite that resulted in a packaging material with a synergistic antimicrobial effect against Escherichia coli and Candida albicans. Chitosan itself shows a biocide activity, and this effect was highlighted in ultrathin fiber mats of zein/chitosan blend that prevented cell growth of Staphylococcus aureus (<1 Log CFU/mL) [79]. Furthermore, the antimicrobial activity of the chitosan fibers was stronger and more efficient than the neat zein fibers [79].
A different strategy was proposed by Huang and co-workers [80] who prepared protective membrane for pork made of a negatively charged cellulose acetate (CA) electrospun fiber mat modified with multi-layers of positively charged lysozyme, chitosan, and organic rectorite composites, and negatively charged sodium alginate via layer-by-layer (LBL) self-assembly technique. As these membranes present higher surface area, they can improve the contact and the interaction between the composites (lysozyme, chitosan, and organic rectorite) and the bacteria, besides its effect against food spoilage and pathogenic microorganisms by damaging the cell walls of E. coli and S. aureus [80].
3.1.3. Smart packaging
Besides its antimicrobial activity and the enhancement of food shelf life, the food protection from warm temperatures is required during their transportation and storage. Alterations caused by high temperatures can lead to detrimental changes in food products such as crystal ice growth and acceleration of chemical reactions. To overcome this issues, Pérez-Masiá and co-workers [81] developed a core-shell zein nanofiber mat that encapsulated a phase changing material, dodecane paraffin (transition temperature at −10 °C), to keep the low temperature constant during food transportation and storage. The nanofiber mat was able to manage and control the heat during 20 heating-cooling cycles, and this composition could be applied to smart packaging materials with the ability to enhance the food storage by maintaining the desired temperature for more time [81].
3.2. Enzyme immobilization
Enzymes can be used as green catalysts with a high degree of specificity (regio- and stereoselectivity) and in a wide range of application as biotechnological tools in the chemical, food, cosmetic, and pharmaceutical industries [[82], [83], [84]].
Some properties of enzymes include environmentally friend biomass production, catalysis of specific chemical conversions, and catalysis of reactions under mild conditions of temperature and pressure [80,82].
However, besides these interesting properties, enzymes lack on stability when they are prepared in solution what entails their ability for reuse since they usually have a defined cycle of recycling and reuse [82,83]. Moreover, due to their particular synthesis and storage conditions, enzymes belong to a group of high-cost chemicals [82,83,85]. In this sense, the immobilization or attachment of enzymes to a robust and inert carrier/support is an interesting strategy that allows them to be recycled through multiple cycles of the batch [83,84]. Particular attention has been giving to nanofibers prepared by electrospinning for enzyme immobilization due to their high porosity, high specific surface area, inter-fiber porosity, a low hindrance for mass transfer, easy handling, and good mechanical strength of the fiber mat [84].
The most important aspect in enzyme immobilization is to retain their natural function with minimal impact on the tertiary structure or active site of the enzyme [82,84]. There are three main types of enzyme immobilization: a) physical adsorption, b) covalent attachment and c) encapsulation [[82], [83], [84]]. All the immobilization method presents advantages and disadvantages because immobilization is usually accompanied by changes in enzymatic activity, optimal pH, temperature, and stability [82].
The physical adsorption of enzymes is the simplest way of immobilization. However, this method does not prevent the enzyme leaching, what can be reflected by low cycles of reusability compared to the covalently immobilized enzymes. For example, Siqueira and co-workers [86] electrospun PLA/chitosan fiber mats and immobilized the lipase by dipping the film into the enzyme solution. The nanofibers presented a dimple morphology that favored the enzymes' cluster and, consequently, presented high enzyme activity retention (maximum of 120% compared to the free lipase). On the contrary, the lipase-immobilized fiber mats were able to be reused only for five cycles [86]. Sathishkumar and co-workers [87] found similar reusability results when they immobilized laccase in PLGA fiber mats to be used in the transformation of diclofenac before its biomagnification in the food chain. Despite the high rate of activity of the immobilized laccase (82% compared to the free enzyme), and their better storage condition, pH, and thermal stability, the laccase-immobilized fiber mats were only able to be used for three cycles during the diclofenac transformation [87].
On the other hand, covalent attachment is an efficient method to prevent the enzyme leaching. It can provide a permanent linkage of enzyme amino acid residues ( NH2,
NH2,  CO2,
CO2,  SH) to organic functional groups that belong to the support [[82], [83], [84]].
SH) to organic functional groups that belong to the support [[82], [83], [84]].
The crosslinking with glutaraldehyde have shown excellent results in keeping enzyme activity and preventing the leaching. For example, Moreno-Cortez and co-workers [88] successfully immobilized papain enzyme in polyvinyl alcohol (PVA) nanofibers mat by chemical crosslinking with glutaraldehyde vapor treatment, achieving about 88% of catalytic activity and keeping 40% of the initial catalytic activity after 14 days of storage. In a similar approach, Fazel and co-workers [89] were able to immobilize horseradish peroxidase in PVA/bovine serum albumin (BSA) nanofibers mat via glutaraldehyde vapor treatment. They demonstrated that the immobilized horseradish peroxidase tolerated higher concentrations of hydrogen peroxide that the free enzyme and that its residual activity was about 73% after 11 cycles of use [89].
Using a reactive electrospinning process, Tang and co-workers [90] immobilized hyperthermophilic enzymes (α-galactosidase and β-glucosidase) in PVA nanofibers. During a single step procedure, the enzymes were immobilized by crosslinking with glutaraldehyde solution at the same time as nanofibers, in a way that effectively entrapped the enzymes and prevented leaching. Furthermore, the immobilized hyperthermophilic enzymes presented an enhanced thermostability [90]. Concerns are that sometimes the covalent immobilization can hinder the enzyme active site and will require the use of organic solvents, as well [82,90]. Furthermore, pH changes could denature the biomolecule [82].
In turn, the encapsulation of enzymes has been an effective immobilization technique as it can control the enzyme leaching and, at the same time, allows the enzyme active sites to remain free and extend its cycles of reuse [82,84].
The enzyme trypsin was entrapped into PCL nanofibers through a methodology that consisted in electrospinning an emulsion composed of an aqueous phase (enzyme solubilized in a buffer solution) and an oil phase (PCL solubilized in chloroform/dimethylformamide) [91]. The nanofibers did not show a core-shell structure but a preferred location of the enzyme near to the fiber's surface [91]. Although this system showed minimal enzyme leaching, the better activity retention was 66%, with stability retention of about 59% after five cycles of reuse [91].
3.3. Tissue engineering or regenerative medicine
Tissue engineering makes use of three-dimensional substrates called scaffolds to support cell cultures. Some bio-based and natural polymers have been applied as electrospun scaffolds in a variety of compositions and morphologies; among them include PHA's, PCL, collagen, gelatin, and hyaluronic acid. Developments using cellulose acetate as a component of scaffolds have shown innovation concerning the microarchitecture of the biomaterial. Porosity control and surface modification have taken much attention because of their significant influence on the tissue bridging and resistance to the cell adhesion [[92], [93], [94]]. Rodríguez and co-workers [92] developed a CA nanofibrous scaffold with controlled microarchitecture using a computer-assisted design approach that allows obtaining a fiber mat with pores between 50 and 300 μm, by post-treatment processing with a CO2 laser intervention and phosphate buffer solution, without damaging the surrounding area of the mat. After that, the material was submitted to a saponification procedure with the aim to regenerate the material to cellulose and allow the growth of calcium phosphate crystal for further osteoblast cell culture. The modified surface allowed the calcination by calcium phosphate and resulted in similar diffraction pattern to hydroxyapatite. Both mineralization and porosity control had a positive influence on cell behavior, allowing a better adhesion and stretch than the scaffold without surface modification.
Polyamide-6 (PA-6) presents structural and molecular similarity to the collagen present in bones, besides its suitable mechanical properties and nontoxicity [[95], [96], [97]], what call attention to using PA-6 as polymer base for functionalized scaffolds [98]. Pant and co-workers [98] developed a PA-6 mineralized scaffold through electrospinning PA-6/lactic acid, which has potential as filler or even artificial bone. During the electrospinning process, the lactic acid was evaporated and remained on the surface composing the sheath, as the PA-6 act as a base polymer (core). For modification with calcium lactate (CL), the fiber mats were embedded in a 0.1 M Ca(OH)2 solution and later dried for further use. For mineralization, both PA-6 and PA-6/CL fiber mats were incubated with simulated body fluid for ten days. Energy Dispersive X-ray (EDX) coupled to a scanning electron microscope confirmed the calcium deposition on the surface of the PA-6/CL nanofibers and indicated an increase of the deposited calcium as four times greater than that deposited calcium on a PA-6 fiber mat.
Goes and co-workers [99] investigated the feasibility of electrospinning PBAT with montmorillonite nano clay (MMT). The PBAT/MMT nanofibers mats presented smaller average fiber diameters than to the pure PBAT fiber mats, providing a higher surface area for the composite nanofibers mat. Through transmission electron microscopy, it was possible to observe MMT lamellae distributed over the nanofibers surface. The PBAT/MMT fiber mats were not cytotoxic for SkHep cells (human hepatoma cell line) and fibroblasts from cardiomyocytes. Also, both pure PBAT and PBAT/MTT fiber mats presented a pleasant environment for growth and proliferation of fibroblasts from cardiomyocytes.
Pure PBS and PBS composite fiber mat scaffolds were firstly produced by Zhang and co-workers [100]. PBS was electrospun with different concentrations of wollastonite (CaSiO3) nanowires and submitted to incubation in simulated body fluid (SBF). After the incubation, it was observed the formation of microspheresof apatite on the surface of the PBS nanofibers, indicating a biomimetic production of scaffolds that can be applied for bone regeneration.
In a different approach, Sutthiphong and co-workers [101] synthesized a PBS modified by the addition of 1,6-diisocyanatohexane and electrospun the copolymer, obtaining a nanofibers mat with an average fiber diameter of 170 nm. The scaffold was tested for cytotoxicity and bone cell culture using mouse fibroblasts (L929 cell line) and human osteosarcoma cells (SaOS-2). The fiber mats did not show cytotoxicity and presented high response on cell attachment and proliferation. The cell response on the modified PBS fiber mats was superior to that of solvent-cast films with the same polymer composition.
PET shows interesting mechanical properties and biological affinities; however, its high hydrophobicity restricts its use in tissue engineering. An alternative to improve PET application as a scaffold is to functionalize the surface of the biomaterial with molecules with hydrophilic character. Recent publications have shown the ability to electrospun PET with biomolecules like chitosan [102] and collagen [103]. The use of functionalization-anchorage method allowed manipulating the properties of the electrospun fiber mats as the inclusion of biomolecules changed the key features, like fiber average diameter: the diameters increased with the increase of chitosan content [102], or a population of thinner fibers was observed increase as the amount of collagen increased [103]. The presence of biomolecules in the electrospun fiber mats changed its hydrophobic nature acting as an enhancer for penetration and cell colonization as well as leading to high levels of cell adhesion, spreading and proliferation [102,103]. The combination of mechanical strength and biofunctionalization of electrospun PET nanofibers mats have also led to its surface engineering with gelatin, what provided a suitable scaffold for endothelial cells to spread and proliferate [104].
Following the same ideation for vascular tissue engineering, however, by using a different approach, Merkle and co-workers produced a core-shell PVA/gelatin nanofiber mat where PVA was the core and gelatin was the sheath. Human umbilical vein endothelial cells (HUVE) could attach to multiple sites along the nanofiber mat surface, responding according to the mechanical properties of the scaffold: the cells' spreading increased as the mechanical stiffness of the nanofiber mat increased [105].
Recently, Stafiej and co-workers [106] reported the use of PCL/poly glycerol sebacate (PGS) and PCL/chitosan nanofiber mats as scaffolds for human corneal epithelial cells (HCEp) to be used as a replacement of the human amniotic membrane and improve wound closure in defects of the human cornea. The HCEp were able to adhere and proliferate with oriented growth in the scaffolds showing aligned nanofibers orientation, what demonstrated the feasibility of the electrospun PCL/PGS and PCL/chitosan as potential biomaterials for the ophthalmological application [106].