1. Introduction
Encapsulation techniques enable the development of composite particles, which typically have a core material of interest coated by a secondary material. Based on the properties of the coatings, encapsulation can impart properties of controlled core material release, protection from non-specific chemical interactions, ease of handling and transport, and facile separation from matrices to the overall composite particle [1]. Microencapsulation, which is the preparation of encapsulated composite particles in the micron size range, has found widespread use in several industries such as pharmaceuticals, personal care, medical, aromatherapy, paint, oil & gas, textiles, paper, electronics, and food systems [2,3]. For instance, magnetic PLGA-loaded microspherescontaining the cancer drug 5-fluorouracil are an active target delivery system that increases the accuracy of tumour targeting and delivers drugs at an optimized rate at the specific site [4]. In the food packaging and preservation domain, essential oils are highly desirable because of their antioxidant and antimicrobial properties, protecting food from spoilage. However, their incorporation into food is challenging, especially for low-fat products, due to their hydrophobic nature, strong aroma, and flavour. Encapsulation of essential oil (emulsion type encapsulation) impacts these organoleptic properties, acts as an alternative solution to synthetic preservatives, and maintains the quality of meat, fish, dairy, fruit, and vegetable products [5,6]. Nano encapsulated particles have also been used in similar applications as they show better core material encapsulation efficiencies, sustained material release, and improved solution stability compared to microencapsulated particles [7,8]. Based on their desired application, encapsulated particles can be prepared in a dry form as free-flowing powders, as slurry/wet filter cake, beads, fibres, or emulsions [9,10]; [11].
Due to their wide-ranging applications, review articles related to encapsulation techniques and their uses [[12], [13], [14]] have been published in various research domains aiming to highlight and critique the progress in their respective fields thus far. For instance, in the pharmaceutical industry, encapsulated drugs provide controlled and targeted delivery, improved stability, and bioavailability . Recent development in nanoencapsulation and delivery of natural bioactives or pharmaceuticals using chitosan scaffolds was reviewed by [15].The article focused on the mechanisms of controlled in vitro/in vivo release in various biological and physiological environments and different modification techniques of chitosan-based nanocarriers for biomedical applications and medical uses [16].provided a detailed review on targeted and controlled releaseof essential oils using nanoencapsulation methods. The article presented the advantages of encapsulation in overcoming the challenges associated with solubility, rapid volatilization and degradation of essential oils in harsh environments. The article also provided an overview of promising strategies for encapsulating essential oils.
A recent review of potential micro- and nanoencapsulation systems for anthocyanins reported by Ref. [17] showed that biopolymer-based nano-encapsulated particles, complex coacervates, and tocosome provide better bioavailability and stability than other encapsulated systems. Preserving aroma and flavor [18] is essential in the pharma and food industries [19]. reported on the significant advantage that encapsulation presents in preserving flavor and aroma. The article reviewed the benefits of encapsulation in overcoming high volatility, controlled release, and bioavailability limitations. In the food industry, nanoencapsulation of food bioactive components and related processes were reviewed by Ref. [20]. In the review, the authors examined how the bioavailability of nutrients, nutraceuticals, and other food components was preserved due to encapsulation, and summarized some encapsulation techniques such as emulsification, coacervation, electron precipitation, and electrospraying [21]. also reviewed recent approaches to use various food components for nanoencapsulation. The paper discussed various nanoencapsulation techniques and innovative applications of nano-encapsulated phenolic compounds, antioxidants, food colorants, essential and mineral oils, fragrances, antimicrobial agents, and vitamins as a nanocarrier in the food sector.
Overall, the review articles highlight the growing importance of encapsulation techniques in different fields such as pharmaceuticals, nutraceuticals and food. In recent years, use of micro- and nano-encapsulation techniques has seen growing interest within different environmental domains as well, especially in water, energy, and agriculture [9,10]. The use of encapsulation techniques hold strong potential to improve several processes and functionalities in environmental applications, such as those in water treatment, renewable energy and agricultural processes. However, a comprehensive review of encapsulation approaches in the environmental domain is lacking. An in-depth review highlighting the benefits of micro and nano encapsulation in the environmental field will greatly benefit researchers in developing more innovative and sustainable approaches in applications such as pollution mitigation, energy storage, and plant growth. This review paper seeks to bridge this knowledge gap and reviews the various encapsulation methods, the different components of encapsulated particles, as well as provides a detailed discussion on various environmental applications of micro and nano-encapsulated particles investigated in literature thus far.
2. Encapsulation components
Encapsulated particles typically consist of two components, (1) the core and (2) the coating material/shell. Core materials, which are the active components to be coated, can exist in different physical states, including liquid, solid, or gas.
Solids are dispersed in a polymeric solution, followed by polymer precipitation using the solid-phase separation method [22]. Also, solids can be dissolved in compatible solutions depending on the core solubility. If the core is soluble in an organic solvent, both the core and the coating materials are dissolved in it, followed by emulsification and evaporation of the organic solvent to cause nano-precipitation via the single-emulsion solvent evaporation technique [23]. For water-soluble core, the solid is dissolved in water and then emulsified. Liquid cores can be emulsified, whereas the gas cores can be adsorbed on an inert solid and thereafter encapsulated as a solid core [24].
The coating material/shell is the inert polymeric substance used to coat the core materials with a desired thickness. These materials should be compatible and non-reactive with the core material and provide desirable properties like stability, strength, flexibility, impermeability, and non-hygroscopicity. Some commonly used coating polymers are (1) natural polymers such as polysaccharides (alginate, chitosan, agarose, hyaluronic acid, dextran, etc.) and those which are protein based (albumin, gelatine) (2) synthetic polymers (poly-E-caprolactone, polyethylene glycol, polylactic-co-glycolic acid, etc.) and (3) sensitive polymers which are polymers that are designed with biologically responsive moieties in order to change their physical and chemical properties in response to exogenous and endogenous factors such as pH, temperature, etc. [25]. Typically, along with polymers, coating compositions include plasticizers, coloring agents (in food and pharmaceutical applications), resins, waxes, lipids, and release rate modifiers [26].
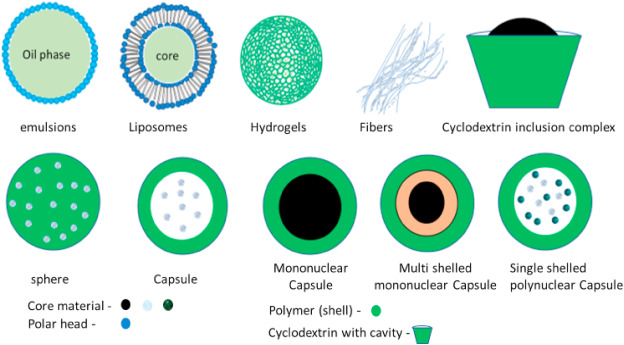
Encapsulated particles can be classified into two main groups based on the dispersion of the core material: matrix and vesicular (Fig. 1). Matrix systems are those in which the active component or core is physically and uniformly dispersed. In contrast, vesicular systems are those in which the core material is confined to a cavity encircled by a polymer membrane (also called capsules) [24,27,28]. Both vesicular and matrix systems can be denoted by different terms depending on their composition, shapes and coating materials, and fabrication techniques(Fig. 2).
 Fig. 1. Visual illustration of encapsulation components.
Fig. 1. Visual illustration of encapsulation components. Fig. 2. Visual illustration of different types of encapsulated particles [29,30].
Fig. 2. Visual illustration of different types of encapsulated particles [29,30].3. Classification of Encapsulation techniques
The selection of an encapsulation technique depends on the physio-chemical characteristics of core materials and their intended application. Based on these considerations and other cost-related and environmental reasons, encapsulation methods are classified into different categories, as illustrated in Fig. 3.
 Fig. 3. Classification of Encapsulation techniques(Krishnakumar and Nair 2018 [24];
Fig. 3. Classification of Encapsulation techniques(Krishnakumar and Nair 2018 [24];3.1. Physical methods of encapsulation
The physical methods are those involving physicochemical and mechanical reactions in particle formation as discussed below [9,31].
3.1.1. Coacervation/phase separation
Coacervation/phase separation is one of the oldest and most widely used techniques of encapsulation [32]. Generally, the process consists of three steps accompanied by continuous agitation: (1) formation of the reaction medium (which is a solution of the coating polymer where the core is suspended), (2) coating material precipitation and deposition, (3) rigidization/solidification of the deposited coating material [33].
In step 1 mentioned above, the polymer is fully solvated using a suitable solvent, thereafter the core material is added with continuous stirring to form the dispersion. The solvent for the polymer is selected so that it does not dissolve the core [34]. In step 2, the coating material (polymer) is separated from the solvent to coat the suspended core material by applying one of the following methods.
-
•
Changing the temperature and pH of the polymer solution.
-
•
Addition of nonsolvent.
-
•
Addition of salt (salting-out method).
-
•
Addition of incompatible polymer to the polymer solution.
-
•
Inducing polymer-polymer interactions.
- •
The separated polymer is deposited over the core material with the aid of controlled physical mixing of the polymer solution and the core material in the solvent. The polymer adsorbs at the interface formed between the core and solvent, which is promoted by reducing the total interfacial energy of the system [32].Step 3 consists of the addition of cross-linking agents or the use of thermal or desolvation techniques to facilitate the solidification or hardening of the formed coat over the core material [35,36].
3.1.2. Ionotropic gelation method
Ionotropic or ionic gelation is a commonly used method to create hydrogels. In this approach, the hydrophilic polymer-based interaction of a cationic or anionic polymer with an oppositely charged molecule creates encapsulated particles (Fig. 4) [37].
 Fig. 4. Schematic representation of ionic gelation method reused from Ref. [38] under creative commons license.
Fig. 4. Schematic representation of ionic gelation method reused from Ref. [38] under creative commons license.Biodegradable polymers like chitosan, sodium alginate, gelatine, carboxymethylcellulose, and gellan gum are often used for this technique [28,39,40]. These polysaccharides produce gels in an aqueous solution of oppositely charged ions at a concentration below the gel point. The formed three-dimensional, hydrophilic matrix is capable of imbibing large volumes of water or fluids. This method is non-toxic (organic solvent-free method) and highly suitable for encapsulating hydrophilic core materials [41,42]; [43]. It is simple, economical, and ideal for low molecular weight core materials, however, its poor mechanical stability in acidic conditions is one of its drawbacks [44].
Hydrogel beads with sodium alginate using divalent cations of calcium, barium, and stannous salts and polyvalent cations like Al3+ have been reported in the fields of pharmaceuticals and wastewater applications. Modified ionotropic gelation techniques or complex coacervation involving two polymers like chitosan and alginate with calcium cross-linking methods are also widely reported in drug encapsulation applications [40]. For instance, Desai et al. (2016) reported modified ionic gelation with chitosan nanoparticle preparations like inclusion complexation [43].
Ionic gelation can be achieved by two different methods.
3.1.2.1. External gelation method
In this method, hydrocolloid solution of charged polymer is added into an oppositely charged ionic solution, and gelation occurs by the diffusion of ions from outside into the hydrocolloid system [45].
3.1.2.2. Internal gelation method
In this approach, an insoluble form of the ion (insoluble salt) is added into the hydrocolloid system of charged polymer, then extruded or emulsified into oil, and subsequently acidified to release and dissolve the ion that cross-links with the polymer and forms hydrogel beads or microparticles [46,47].
Both methods require a two-step process involving droplet formation and hardening. The particle size distribution and shape are developed during the droplet formation step. Techniques used for droplet formation include droplet extrusion, spraying, electrospraying, and emulsification [48,49]. However, hardening of the droplet occurs by changing the temperature, pH of the medium or by adding an immobilizing agent or cross-linking agent.
3.1.3. Solvent evaporation/extraction techniques
This method is applicable to encapsulate hydrophilic and hydrophobic core materials. It consists of two steps [32,50].
-
1.
Emulsification of polymer in organic solution with aqueous phase/solvent.
-
2.
Evaporation of the solvent and precipitation of the polymer.
During the emulsification step, an organic/volatile solvent is used that dissolves both the coating polymer as well as the core material to be encapsulated. The coating polymer/core material solution is then added into an aqueous phasewith continuous agitation using probe sonicator or high shear mixer to form oil in water (O/W) emulsion (Fig. 5). In a simple evaporation method, distilled water without additives is used as an aqueous phase, whereas, in complex emulsification processes, additives like surfactants or emulsifying agents are added to the aqueous phase to produce a stable emulsion. Once the stable emulsion is formed, the organic solvent is evaporated either by reducing pressure, continuous stirring in a fume hood, solvent extraction, or by a heating process, if necessary. Nanospheres formed by the evaporation of the organic solvent can be used in suspension form, coated onto a substrate, or collected as free-flowing powder. The formed nanoparticles are separated by ultracentrifugation, washed with distilled water to remove the free non-encapsulated core material or the remaining additive residues, thereafter dried by freeze-drying and stored as a freeze-dried powder [51]; . The choice of organic solvent and coating polymer greatly influences this technique as well as nanoparticle properties. A suitable solution should be able to dissolve the selected polymer, be poorly soluble in the external phase, present a volatile nature, low boiling point and low toxicity [52]; [40].
 Fig. 5. Schematic representation of solvent evaporation technique.
Fig. 5. Schematic representation of solvent evaporation technique.3.1.4. Supercritical fluid assisted encapsulation technique
Supercritical fluids are highly compressed gases that exist as both liquid and solid above their critical temperature and critical pressure [40,53]. A small change in its temperature and pressure near the critical point causes a significant variation in the density of supercritical fluids. Water and carbon dioxide (CO2) are commonly used as supercritical fluids in laboratory and industrial applications due to their eco-friendly nature.
SCO2 (supercritical carbon dioxide) is widely applied for encapsulation techniques in food and pharmaceutical applications. The unique properties of gas like viscosity, liquid-like density, excellent solubility, non-toxicity, non-flammability, microbial inactivation, high miscibility, and diffusivity, as well as cost-effectiveness make SCO2 an excellent solvent for encapsulation [[53], [54], [55]] SCO2 can be used in different roles during the encapsulation process such as a solvent, antisolvent, foaming agent, solute, and drying enhancer. Based on its role, methods involving SCO2 can be classified as shown in Table 1.
| Technique | Steps | Reference |
|---|---|---|
| 1. Rapid expansion of supercritical solutions (RESS). | Core material and coating ingredients are dissolved in SCO2 (acting as solvent). Depressurization through a narrow orifice leads to expansion and converts the CO2into the gaseous form. Decrease in solubility leads to the precipitation of coating material and active ingredient creating microcapsules. | [[53], [55]] |
| 2. Rapid expansion of supercritical solution with a nonsolvent (Modified RESS-N). | A suspension of active ingredients is suspended in a SCO2 containing co-solvent (low molecular weight alcohols) and a dissolved polymer is sprayed through a nozzle into atmospheric pressure. This method has been used for the encapsulation of proteins and molecules. | [[9], [40], [55]] |
| 3. Rapid expansion of an aqueous solution (Supercritical spray drying technique) | Core and coating material are dissolved in aqueous solution and sprayed with SCO2 into the high-pressure chamber. SCO2 acts as a drying medium and precipitates out dried nanoparticles. | [55,56] |
| 4. Supercritical antisolvent precipitation (SAS) | An organic solution is sprayed into SCO2 that acts as an anti-solvent. This process leads to the precipitation of microcapsules. | [55,57] |
| 5. Particles from Gas saturated solutions (PGSS)/Supercritical melt-micronization (ScMM). | A gas saturated solution is produced by mixing SCO2into a solution or molten mixture of a core and coating material and expanded through a nozzle into a spray chamber at atmospheric pressure. The rapid change in temperature due to the Joule-Thomson effect results in the solidification of polymer on a core material. | [55,57,58] |
| 6. Supercritical Solvent Impregnation (SSI) | Adsorbent materials such as polymers, cyclodextrins, and inorganic porous materials were soaked into SCO2core material dispersion. Adsorption of core material on the surface of this carrier is precipitated out by depressurization of SCO2. | [57,59] |
| 7. Supercritical fluid extraction of emulsions (SFEE). | An emulsion is prepared by mixing the organic phase (coating and core material dissolved) with the aqueous phase including additives/surfactants. SCO2 is used to extract the organic solvent from the emulsion. Supersaturation results in precipitation of coated core material and the nanoparticles are collected from the aqueous solution. | [[53], [55]] |
3.1.5. Sol-gel method
The sol-gel method involves the formation of a colloidal suspension (sol) followed by gelation of the sol to form a network in an external liquid phase (gel). This process consists of 4 steps [9,60].
-
•
Preparation a solution using core and polymer.
-
•
Formation of suspension (Sol).
-
•
Conversion of sol to gel form (Gelation).
-
•
Solidification
The precursor usually used for synthesizing colloidal suspension consists of metal compounds, such as metal alkaloids, metal chlorides, and acetyl acetonesas oxides, water used as hydrolysis agents, and alcohol used as a solvent. Acid and bases are used in some reactions as catalysts. Hydrolysis and polycondensation reactions are generally involved in the sol-gel process. Metal compounds readily undergo hydrolysis and polycondensation reactions (alcohol-based condensation and water-based condensation reactions) near room temperature and produce sol in which polymers or fine particles are dispersed. The final products formed in this method usually are wet gels, dried gels, heated gels, glass, and ceramics [61].
3.1.6. Liposomal encapsulation
A liposome is a spherical-shaped vesicle composed of one or more phospholipid bilayers surrounding aqueous molecules. It can be created from cholesterol and natural, non-toxic phospholipids, with a structure closely related to the human cell membrane [62]. Phospholipid bilayer consists of a hydrophilic head and hydrophobic tail, where the polar head is oriented in the form of interior and exterior aqueous phases. Due to the hydrophilic and hydrophobic character of its structure, liposomes are commonly used as a nanocarrier in drug delivery systems [63]. Based on liposome size and several lipid bilayers, liposomes can be classified in to four categories as shown in Fig. 6.
 Fig. 6. Schematic diagram of liposomes classification [64].
Fig. 6. Schematic diagram of liposomes classification [64].In unilamellar vesicle-type liposomes, the aqueous phase is enclosed in a single phospholipid bilayer. In multilamellar vesicle, several layers of phospholipids are arranged as structures similar to an onion in which layers of water separate each phospholipid layer. Oligolamellar vesicles and multivesicular vesicles are different in their number of compartments of phospholipid bilayers. Generally, liposomes are created by dissolving the raw lipids in an organic solvent (ethanol/chloroform) and slowly injected to an aqueous solution of the material to be encapsulated at 55 °C–65 °C or under reduced pressure. The consequent removal of the organic solvent under vacuum leads to the creation of liposomes. Generally, the liposome preparations include the following sequential steps [[42], [62], [65]].
-
•
Phospholipids are dissolved in an organic solvent (ethanol/chloroform).
-
•
Heating/drying
-
•
Dried film is dispersed in medium (mostly buffer solutions).
-
•
Sizing step (use of different sonication methods)
-
•
Liposome evaluation/characterization.
Encapsulation of active material loaded into the liposomes is based on the nature of the core material. If the material is lipophilic, it should be solubilized in the organic solution of the constitutive lipid and evaporated to form a dry film with active core material, followed by dehydration and purification. In contrast, hydrophilic materials are entrapped by an aqueous solution or by the addition of a core or core solution at some stage of the manufacturing process. Generally, liposomal loading methods are categorized as follows [66,67].
-
a)
Active loading method: Core that displays both hydrophilic and lipophilic solubility and compounds with an ionizable group can be entrapped into the liposomes after forming intact vesicles.
-
b)
Passive loading method: In this method, the core material is entrapped into the liposomes before or during the manufacturing process. In general, hydrophilic compounds are distributed homogenously in the aqueous phase, whereas hydrophobic compounds are retained inside the lipid bilayer of liposomes, respectively. Different types of passive loading methods are shown in Fig. 7.
 Fig. 7. Classification of different methods of liposomal preparations.
Fig. 7. Classification of different methods of liposomal preparations.
3.1.7. Molecular inclusion complexation with cyclodextrins
Inclusion complexes are molecular compounds in which one chemical compound has a cavity (host molecule), which accommodates or spatially encloses a guest molecule, and a complex of the host-guest structure is formed (Fig. 8) [[68], [69], [70], [71]]. Inclusion complexation with cyclodextrin molecules is extensively used for encapsulation techniques. Cyclodextrins are cyclic oligosaccharides that belong to the category of carbohydrates. Naturally occurring cyclodextrins are three types α, β, and γ and are commonly called Schardinger sugars. Geometric compatibility and chemical properties of guest molecules are dependent variables for producing inclusion complexes. The compound having a size that is compatible with the dimensions can penetrate the cyclodextrin cavity. Less polar guest molecules rapidly form inclusion complexation with cyclodextrins. Different techniques used for producing inclusion complexation with cyclodextrins are discussed in Table 2 [72] [73].
 Fig. 8. Inclusion complexation with cyclodextrins [74].
Fig. 8. Inclusion complexation with cyclodextrins [74].Table 2. Inclusion complexation methods with cyclodextrins.
| Technique | Process | References |
|---|---|---|
| 1.Blending | Cyclodextrins and core are mixed by trituration method and pass through a suitable sieve to get the desired particle size. | [68] |
| 2.Kneading | Paste of cyclodextrin in water or alcoholic water is mixed with the active material. The kneaded mixture is dried and passed through a sieve. | [68,75] |
| 3. Co-precipitation and neutralization | The core is mixed with cyclodextrin solution under controlled parameters; the precipitated content is filtered through a vacuum and dried at room temperature. In the neutralization method, the core is dissolved in an alkaline solution and mixed with an aqueous solution of cyclodextrin, followed by the addition of acid that favors the precipitation of the inclusion compound. | [68,76] |
| 4. Solvent evaporation | The core and cyclodextrins are dissolved separately in two miscible solvents,and finally, the solvent is evaporated under a vacuum to obtain a solid powdered inclusion compound. | [68,76] |
| 5. Milling | Core and cyclodextrins are mixed and introduced in an oscillatory mill. | [[68], [69]] |
| 6. Spray drying | It is a common technique used in pharmaceuticals to produce a dry powder from a liquid phase. The mixture of active material/core and cyclodextrin solution passed through a narrow orifice and dried under thermal stress. | [28,68] |
| 7. Freeze drying | The solvent is eliminated from the solution through a primary freezing and subsequent drying of the solution containing both core and cyclodextrin. | [68] |
| 8.Microwave irradiation | Active ingredients and cyclodextrin are mixed with water and organic solvent. The reaction mixture should be placed into a microwave oven at 60 °C to initiate the reaction. After completing the reaction, an adequate volume of solvent is added to the reaction vessel, and the precipitate is filtered out and dried in an oven at 40 °C for 48 h. | [68,77] |
| 9.Supercritical antisolvent system | The core and cyclodextrin are dissolved in a good solvent then the solution is sprayed into supercritical fluid anti-solvent (SCO2), the mixture becomes supersaturated favoring the precipitation of the solute and the solvent is eliminated with the supercritical fluid flow. | [[68], [78]];[79]. |
3.1.8. Micelle based encapsulation techniques
A micelle is an aggregate of surfactant phospholipid molecules dispersed in a liquid, forming a colloidal suspension. Micelles have a lipophilic core (tail) and hydrophilic shell (head) or, hydrophilic core with lipophilic head (reverse-micelle) as shown in Fig. 9 [80].
 Fig. 9. Different type micelle orientation in encapsulation.
Fig. 9. Different type micelle orientation in encapsulation.Surfactant molecules, which are made up of polar, ionic, or non-ionic head groups self-assemble into micelles in aqueous solutions [81]. Micelles form only when the concentration of surfactant is greater than the critical micelle concentration (CMC), and the temperature of the system is greater than the critical micelle temperature.
Core materials are generally encapsulated into micelles by three mechanisms: chemical conjugation, physical entrapment, and polyionic complexation. In chemical conjugation, a core is chemically conjugated via a pH or enzyme-sensitive linker to the core-forming part of the copolymer. The physical entrapment or solubilization of core within block copolymer is preferred for hydrophobic core materials. Physical entrapment method includes: emulsification (o/w), dissolution techniques, solvent evaporation methods, solvent casting method, and dialysis [82]. Polyionic complexation is used for incorporating charged core materials into block copolymer micelles. Usually, an oppositely charged ionic segment of block copolymers is selected for it, and entrapment depends on the electrostatic interaction between the molecules. Micelle-based encapsulation is commonly used in pharmaceutical industries for entrapping poorly water-soluble drugs and for targeted drug deliveryapplications.
3.1.9. Air suspension (wruster) methods
The air suspension method also called as wruster method [32], is characterized by the location of a spray nozzle at the bottom of the fluidized bed. The particles are moved with an air stream and the nozzle spray atomizes droplets of coating solution concurrently with the particle flow, depositing droplets on the surfaces of the particle. Organic or aqueous coating solutions evaporate as the particles move through the expansion chamber, leaving non-volatile coating formulation ingredients on the particle surface as part of the film coat. The particle size produced by this method depends on the properties of core material, quantity and concentration of coating material, inlet and outlet air temperature, as well as spray nozzle size and spray setting process [83,84].
3.1.10. Spray drying and spray congealing method
This method produces dry nano-encapsulated powder from a liquid or slurry by rapidly drying with a hot gas. In this process, the active core material is dissolved or suspended in a polymer or coating solution and entrapped in the dried particles. Essential steps included in the spray drying process are below [83,85].
-
•
Heating of gas used for the drying process
-
•
Droplet generation/spraying or atomization process
-
•
Droplet drying
-
•
Dried particle collection
The method is commonly used for the encapsulation of thermolabile material. The spray drying and congealing process are similar in the process but the difference in coating solidification principle. In the spray drying method, the coating solidification happens by rapid evaporation of the solvent in which the coating material is dissolved. Whereas in the spray congealing method, the coating and core material mixture is introduced into a nonsolvent, and solvent from the coated mixture is removed by rapid extraction or evaporation methods [31,85,86].
3.1.11. Centrifugal extrusion method
This method is a liquid co-extrusion process using a rotating extrusion head. The device used in this encapsulation technique consists of a concentric feed tube through which the coating material and core material are separately pumped to nozzles attached to the equipment. The liquid core material is pumped through an inner tube (as shown in Fig. 10), and the coating material flows through an outer tube. The entire device is mounted on a rotating shaft so that the head rotates around its vertical axis. As the extrusion head rotates, the core material and coating material are extruded through the concentric orifices of the nozzles as a jet of a core material encased in the coating material. By the action of surface tension, these coextruded jet breaks into droplets and form microcapsules [87]. The particle size formed by this method depends on the rotating speed of the extrusion head. These techniques are widely used in the food and pharmaceutical industries. Starch, carbohydrates, and maltodextrins are commonly used coating materials in food industries, and these methods are extensively applied for the encapsulation of vitamins [40,88].
 Fig. 10. Schematic representation of co-extrusion technique [87].
Fig. 10. Schematic representation of co-extrusion technique [87].3.1.12. Vibrational nozzle method
Vibrational nozzle method or annular jet techniques consist of laminar flowassisted jet created with core (inner jet) and coating material (outer jet) through a nozzle and an additional vibration of the nozzle of the liquid. The vibrational frequency of the nozzle helps to control the droplet size and produce a uniform droplet with a particle size range of micron to submicron. The shell material is hardened by cooling, chemical cross-linking, or solvent evaporation process. Several types of nozzle heads are used in this method to create different size droplet creations [40,89].
3.1.13. Electro spraying/spinning methods
The electro spinning/spraying methods are electric force-driven processes wherein nano-encapsulated fibers and beads are made from a polymer solution. Electrospinning is a nanofibers production method, whereas electro spraying produces nano-encapsulated beads. Basic electrospinning or electro spraying system requires three main parts (Fig. 11)- (a) spinneret which is a needle with a syringe system, (b) high voltage source, and (c) a grounded collector [90,91]. In this method, the core material is dissolved in a suitable solvent and mixed with coating/polymer solution and passed through a spinneret (needle) while applying an electric voltage. Needle -less spinnerets and coaxial methods are also used for encapsulation. In the coaxial method, the core material is dissolved in a solvent, it is connected to the core inlet and polymer solution connected through the axial inlet. Needle-less electrospinning is commonly used in textile applications.
 Fig. 11. Schematic diagram of electrospinning unit (J [92].
Fig. 11. Schematic diagram of electrospinning unit (J [92].Basic principles of electrospinning for producing fibres are [93,94].
-
•
Jet initiation
Jet initiation is a jet that erupts from the tip of the spinneret due to the application of a high electric voltage. A charged molecule in the polymer solution generates a repulsive force, thereby surface tension of the polymer solution reduces, and a Tylor cone is created at the tip. The formation of the Tylor cone occurs when the electric field overcomes the surface tension of the polymer solution [95].
-
•
Bending instability and further elongation
Bending instability of jet begins with a critical voltage condition which causesuniaxial stretching of the erupting jet from the spinneret and leads to its elongation.
3.1.13.1. Solidification/drying of solution jet into fibres and spheres
High voltage and stretching lead to evaporation of solvent between the spinneret and collector. Entanglement between the polymer chains usually prevents the breaking of the jet and produces fibres on the collector. At high voltage conditions, the jet breaks and produces nanospheres rather than nanofibers [96,97]. If the polymer concentration is too low, the viscosity is not sufficient to produce the polymer chain entanglements and creates electro sprayed nanospheres [91,98].
3.2. Chemical methods of encapsulation
The chemical methods are categorized into polymerization and polycondensation reactions. Emulsions, suspensions, or dispersions act as a precursor for both the aforementioned chemical reactions, providing nano capsules with high purity, high uniformity, and dispersible with small particle size distribution [9].
3.2.1. Polymerization method
Polymerization is a chemical reaction in which the monomers of identical units bind together and form a polymer with the aid of a catalyst [92]. Encapsulation by polymerization reactions is categorized in Table 3.
Table 3. Different type of polymerization methods.
| Polymerization methods | Processing steps | References |
|---|---|---|
| 1. In situ polymerization | Polymerization reaction occurs in an external phase, or an external phase side of the interface created by the dispersed core material and external phase. Prepolymer binding step starts at a continuous phase, and it grows and is deposited over the core material. Polymerization reactions continue with cross-linking and produce a solid capsule shell. | [32,40,99]. |
| 2. Interfacial Polymerization | Reacting materials are dispersed in two different phases, polymerization reaction occurs between the interface of those immiscible phases and generates encapsulated capsules. | [40,99]. |
| 3. Matrix polymerization | On the reaction process, the core material is embedded in a polymeric matrix and is converted into an encapsulated particle by the evaporation of solvent from the matrix system or by chemical reaction. | [99]. |
| 4. Emulsion polymerization | In the technique, polymerizing hydrophobic liquid monomers (reacting monomers) in the aqueous phase form stabilized emulsion (o/w) with surfactant at the initial stage of the reaction. Surfactant molecules aggregate in the aqueous phase and form micelles. Monomer molecules diffuse from the monomer droplet to the aqueous phase and bind with the hydrocarbon center of the micelles, and form polymers. | [99,100] |
| 5. Suspension polymerization |
This technique is a heterogeneous radical polymerization process. Liquid or dissolved monomers are suspended in a liquid phase with rapid agitation; thereby, monomers form droplets and act as small bulk reactors. Polymerization crops up inside the droplet, and the product formed is a sphere of polymer. |
[99,101] |
3.2.2. Polycondensation method of encapsulation
Encapsulation by polycondensation is a condensation process in which monomers with two functional groups react with each other or bind together to form a polymer or poly-condensate. It is a form of step-growth polymerization. Common condensation polymers used in this method include polyacetals, polyamide, and proteins [102,103].
Although various encapsulation approaches exist as described above, it is important to outline the limitations and advantages of the techniques. Table 4provides a comparison summary of the same.
Table 4. Advantages and challenges of encapsulation techniques.
| Encapsulation techniques | Advantages | Challenges | Reference |
|---|---|---|---|
| Physical methods | |||
| Coacervation |
|
|
[104,105] |
| Ionic gelation |
|
|
[106] |
| Solvent evaporation |
|
|
[107] |
| Supercritical SCO2 assisted methods |
|
|
[108,109] |
| Sol-gel method |
|
|
[110] |
| Liposomal encapsulation |
|
|
[105] |
| Molecular inclusion techniques | |||
| Blending |
|
|
[105,[111], [112], [113]] |
| Kneading |
|
|
|
| Coprecipitation and neutralization |
|
|
|
| Solvent evaporation |
|
|
|
| Milling |
|
|
|
| Spray drying |
|
|
|
| Freeze drying |
|
|
|
| Microwave irradiation |
|
|
|
| Supercritical antisolvent system |
|
|
|
| Micelle encapsulation |
|
|
[114] |
| Air suspension |
|
|
[115] |
| Spray drying |
|
|
[115] |
| Chemical methods | |||
| Polymerization methods | |||
| In situ polymerization |
|
|
[107] |
| Interfacial polymerization |
Homogenous size particle formation Chemically and physically stable products |
Difficulty in control. | [107] |
| Emulsion polymerization |
Low-cost production method Easy to control |
High technological requirements | |
| Suspension polymerization |
|
|
[107,116] |
| Polycondensation |
|
|
[117] |
4. Environmental applications of encapsulated materials
4.1. Water and wastewater treatment
Use of encapsulated particles is an emerging area of research in advanced water and wastewater treatment processes. Encapsulated materials and nanocomposites facilitate adsorption and degradation of different pollutants in wastewater, promoting sustainable water use [118]. Matrix-based hydrogels, nanofibers, and metal-encapsulated membranes have also been used in surface-enhanced raman spectroscopy-based detection of pharmaceuticals, heavy metals, and other emerging contaminants in wastewater [[119], [120], [121]]. Recycling of adsorbents is an added advantage of encapsulated materials in wastewater application. For instance, alginate based magnetic beads containing cyane×272® have shown to remove cobalt (II) ions from aqueous solution with uptake capacity of 0.4mmol/g−1 and show three times reusability of adsorbent without changing its initial properties [122]. Magnetic nanoparticleencapsulated alginate beads also show removal capabilities toward a wide range of pollutants such as Pb, with 50% Pb removal within 20min [123]. Another study showed 67% of strontium removal from sea water samples [124]. In industrial and textile wastewater treatment area, encapsulated bio adsorbents, i.e. microorganisms encapsulated in polymeric matrices, have been employed to improve their performance. For instance, in studies of encapsulated microorganisms like baker's yeast (Saccharomyces cerevisiae) [125], and Aspergillus Niger [126], removal of textile dyes (Direct Orange 2 GL) and radionuclides (Thorium) have been reported, respectively. Microorganisms have several disadvantages when suspended, such as low-density, low stiffness, and low mechanical resistance and easily undergo solid-liquid separation under high pressure, filtration, and centrifugation. Encapsulation resolves these demerits and provide good recyclability [127]. Based on the type of encapsulated materials and the nature of their application, they can be classified into four major categories: catalysts, adsorbents, sensors, and membranes. Some of these materials and their application in wastewater are represented in Table 5.
Table 5. Application of encapsulated materials in waste water treatment.
| Core | Shell | Application | Encapsulation technique | Particle size | Ref |
|---|---|---|---|---|---|
| Iron oxide | Chitosan | Water purification by bacterial inhibition and removal of various pollutants like dyes (crystal violet, methyl orange), oil (petroleum oil), heavy metals such as copper and led. | Phase separation/coacervation by the addition of NaOH to the mixture of iron oxide nanoparticles and chitosan | 10–20 nm | [128] |
| Chitosan-TPP matrix system. | Removal of Congo Red (Textile dye) | Ionic gelation method – Chitosan is cross-linking with TPP (Tripolyphosphate). | 40–50 nm | [129] | |
| Removal of Hexavalent chromium | 100 nm | [130] | |||
| Natural clay, Phosphate, and activated charcoal | Sodium alginate | Cadmium adsorption | Ionic gelation method – sodium alginate with core materials are ionically cross-linked with divalent calcium ions | Not reported | [131] |
| Silver | Chitosan | Pesticide (Atrazine) removal from drinking water | Chemical cross-linking and precipitation method using microwave irradiation. | 5.4 μm | [132] |
| PolyPyrrol Nanotube | Sodium alginate | Methylene blue dye adsorption | Ionic gelation method using divalent calcium ions. | Not reported | [133] |
| Silver | Sodium alginate and Carbopol | Removal of methylene blue | Silver- sodium alginate Carbopol hydrogel beads prepared by ionic gelation method and silver incorporated hydrogen beads immersed into 2% Mukai maderaspatna plant extract which acts as reducing agent and form silver nanoparticles. | 11–20 nm | [134] |
| Silver oxide | Chitosan | Removal of organochlorine pesticide – permethrin | Coacervation of phase separation by adding NaOH to the chitosan-silver mixture. | 60 nm | [135] |
| Acetylcholine esterase from D. melanogaster. | Egg phosphatidylcholine liposomes | Detection of organophosphorus pesticide – dichlorvos and paraoxon in drinking water. | Liposomal encapsulation by lipid film hydration method. | Not reported | [136] |
| α- Al2O3 | Acrylamide | Adsorbent for wastewater treatment | Polymerization with sol-gel method. | 10–50 nm | [137] |
| EDTA (ethylene diamine-tetra-acetic acid). | β-cyclodextrin | EDTA-β-cyclodextrin inclusion complex used to remove the ciprofloxacin from water. | Cyclodextrin-inclusion complexation method of encapsulation | Not reported | [138] |
| Magnetic nanoparticle | Gum-Arabic | Methylene blue removal from water. | Chemical cross-linking with glutaraldehyde. | 1.03 μm | [139] |
| CdO/ZnO nanoparticle | Polyurathene | Methylene blue degradation | Sol-gel for nanoparticle preparation followed by electrospinning technique. | Not reported | [140] |
| ZnO | PAN | Pollutant degradation | Electrospinning | 100–170 nm | [141] |
| Zeolite | Polyvinyl alcohol (PVA) | Removal of nickel and cadmium from water | Electrospinning |
PVA fibre – 170 nm Zeolite encapsulated –Not reported |
[142] |
| Zeolite | Chitosan/PVA | Removal of hexavalent chromium, iron, and nickel from water. | Electrospinning | 59–70 nm | [143] |
| PAN | Poly-pyrrole/MnO2. | Led adsorption from water | Electrospinning using PAN/poly-pyrrole followed by in situ polymerization. | 200 nm | [144] |
| Charcol from saw dust | Chitosan | Cu II removal from water | Chemical cross-linking using NaOH. | Not reported | [145] |
| Heulandite/Fe3O4 | Chitosan | Cu II and arsenic removal from water. | Ionic gelation method | Not reported | [146] |
| Polyimide | Cellulose acetate | The membrane is used to separate oil from water. | Electrospinning – coaxial method, using Polyimide in DMAc solution used as core and Cellulose acetate in DCM/acetone mixture used as a shell. | 200 nm | [147] |
| ZnO/TiO2nanoparticle | Sodium alginate | Adsorption and photocatalytic effect to remove copper from water | Ionic gelation method | 453–612 μm | [148] |
| Magnetic nanoparticles | Phospholipid vesicle (liposome) | Adsorbents for wastewater containing fatty acids. | Thin layer evaporation (TLE) and reverse phase evaporation (REV) | 207.6 ± 2.3 | [149] |
| Fe3O4 | Polyvinylpyrrolidone (PVP) | Removal of emulsified oil from water. | Solvothermal method. | 90–410 nm | [150] |
| β-Cyclodextrin | Polycaprolactone (PCL) | Removal of dyes like methylene blue and 4-aminoazobenzene | Electrospinning method | Not reported | [151] |
| TiO2 | PVA | Removal of radioactive pollutants (thorium and uranium) from an aqueous phase. | Electrospinning method | Not reported | [152] |
4.2. Energy applications
Encapsulated particles in the energy storage field play a vital role in efficient, clean, and versatile energy utilization and are crucial for exploiting renewable energy. They provide high efficiency, large specific surface area, controlled heat exchange across the capsule shell, and initiate crystallization with small core-size materials. Because of these advantages, encapsulated materials are used significantly in the energy sector [107].
Phase change materials (PCMs) offer a vast range of possibilities in energy sector and are crucial in efficiently utilizing waste heat and solar energy. However, conventional PCMs face several issues, such as low thermal conductivity, high flammability, poor thermal stability, corrosiveness, supercooling, volume and pressure variations during phase transformation, and leakage of molten PCMs into the surrounding of the thermal energy storagesystem, limiting the commercial acceptability of PCMs.
In addition, there are toxicity concerns related to the use of conventional PCMs. Organic PCMs such as vegetable oils and paraffins under food grade categories are environmentally friendly to use. But its high flammability is not suitable for construction applications without proper fire and safety precautions. Upon burning, commercial-grade paraffin or waxes produce harmful toxins that are non-biodegradable and carcinogenic [153]. As for inorganic PCMs, salt hydratessuch as Glauber's salt cause eye irritation and respiratory problems. Accidental ingestion of calcium chlorides can cause serious health problems and cause eye irritation. Magnesium nitrate PCMS is safe to use, but its non-biodegradability has implications for environmental pollution [154,155]..
To overcome these drawbacks, various heating/cooling approaches and performance enhancement techniques have been developed to promote the use of PCMS in the commercial sector. Encapsulation is one of the performance enhancement techniques of PCMs [156]. Hu et al. (2014) [157]developed a novel encapsulated phase-changing material on a paraffin core and carboxymethylcellulose-modified melamine formaldehyde shell by in-situ polymerization. These PCMS provide better flexibility and phase-changing enthalpy of 83.46 J/g with paraffin content of 63.1%. The heat resistance of capsule shells significantly decreased, and the cracking ratio was 11.0% after carboxymethylcellulose modification and providing better system thermal efficiency. Studies by [158].
[158]shows that biodegradable microcapsules containing three different PCMs, hexadecane (paraffin), butyl stearate (ester), and caprylic acid (fatty acid) were encapsulated inside barium crosslinked pectin shell for thermal energy storage applications. Pectin-PCM capsules with an encapsulation efficiency of 83.66 wt%,83.21 wt% and 84.39 wt% containing hexadecane, butyl stearate and caprylic acid respectively, as core, while corresponding melting enthalpies were 184.89 kJ kg−1, 116 kJ kg−1, and 118 kJ kg−1. 25 g of capsules provided thermal buffering of 62 min, 50 min, and 51mins respectively, during discharge experiments. Pectin - PCM-capsules show better thermal efficiency by 70 °C-130 °C compared to core PCMs.
Encapsulated PCM is classified into three types according to particle size (1) Nano-encapsulated PCM 1 nm–1000 nm) (2) Microencapsulated PCM (1 μm–100 μm) (3) Macro encapsulated PCM (greater than 1 mm) [159]. Common techniques used for Nano encapsulated PCM (NEPCM) synthesis include interfacial polymerization, emulsion polymerization, mini-emulsion polymerization, in situ polymerization, and sol-gel methods. However, prevention of particle aggregation is a critical challenge during synthesis. Core-shell ratio, surfactant concentration, and stirring rate greatly influence capsule morphology and aggregation, so optimization of these factors should be considered during synthesis [160].
The inclusion of NEPCMs into a host liquid can be considered as a new type of nanofluid or nanosuspension, and is a novel and practical approach to improving the heat transfer process in the thermal energy storage or release of PCMs [[161], [162], [163], [164], [165]]. These types of fluids are collectively called Latent functionally thermal fluids (LFTF). They have high energy storage capacity than base fluids. However, its subcooling, instability, and high viscosity limit its performance in practical applications. Microencapsulated PCMS (MEPCMS) provide considerable heat capacity enhancement at low mass fraction. But during recycling, these fluids show low performance due to cracking of microcapsules during pumping. Unlike MEPCMs, nano encapsulated PCMs have several advantages such as better suspension stability, larger surface area, and lower cracking rate during pumping. However, the preparation of homogenous and stable nanofluid is one of the technical challenges for practical application. Dispersed micro and nano encapsulated PCMS aggregate in base fluids due to strong van der Waals interaction between nanocapsules. Increased agglomeration rate affects the thermo physical properties of LFTF. Various methods like mechanical agitation, stirring, ultrasonication, addition of surfactants, and different type functionalization of capsule surface improves the thermal conductivity, viscosity, and specific heat of these fluids [166].
Other examples and their applications are mentioned in Table 6.