1. Introduction and background
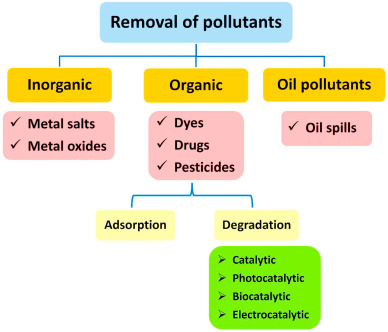
The most precious components for living creatures are water resources and finite resources and it is continuously recovered in water cycles. Recently, water becomes scarce resources, and for a lot of countries supplies already fall short of demanding [1]. Owing to climate changing and growing of population over urban regions, water becomes even scarcer. In addition to, chemical, physical, or biological characters of water were altering, attributing to the continuous presence of pollutants such as organic/inorganic contaminants, pathogenic organisms, heavy metals or other toxic compounds from different resources, making it not safe for the environment and for drinking, so it could be expressed as wastewater [2]. Additionally, standards for the water quality become more stringent owing to the emerging of the pollutants and imposing new scrutiny to the treatment of water and systems of distribution that are widely performed in the developed countries. Therefore, there is an increment in the requirement of affordable and clean water especially to be exploitable for drinking, that could be ascribed as one of the most vital humanitarian goals and it still expressed as a major challenging for last decade [[3], [4], [5], [6]]. The mechanism of removal for different pollutants in wastewater was investigated in literature [7] as suggested in Fig. 1. Whereas, the application of biological treatment, their main disadvantages are slow reaction rates, disposal of sludge and requirement for controllable pH and temperature. Exploitation of nanostructures as catalysts (nano-catalysts) for removal or degradation of organic pollutants has gained considerable interest [[8], [9], [10], [11]].
 Fig. 1. Different supposed mechanisms for removal of pollutants in wastewater.
Fig. 1. Different supposed mechanisms for removal of pollutants in wastewater.This review aims to present unique/quite simple/biogenic methodologies for synthesis of PdNPs and studying their potential uses as nano-catalysts for removal of organic pollutants. The represented review is considered with the recent reports that studied the efficiency of nanopalladium originated from polysaccharides and their applications in treatment of wastewater. By comparing with the conventional techniques of water treatment, the mechanism of catalytic action for nanopalladium was hypothesized in literature [[12], [13], [14], [15], [16]]. The current review also focused on highlighting the nucleation of nanopalladium structures from cost effective biological polymers, i.e., via ecofriendly/green synthetic techniques. Additionally, the basic concept for preparation of nanopalladium@polysaccharide composites is represented, while, it is worthly affirmed that the composition of the nanocompositescontaining biologically originated nanopalladium and eco-friendly polysaccharide/bio-degradable polymers are especially represented. Additionally, Recent investigations of some cutting-edge applications of nanopalladium/polysaccharide composites in the field of wastewater treatmentare also discussed. The recent challenges and the future perspectives in the mentioned topic are also represented in order to show the investigative angles for exploiting of such ecofriendly approaches in fabrication of nanopalladium/polysaccharide composites as well as widening their applicabilities.
2. Green synthesis of nanostructures
In the early stage of nucleation, the metal ion is reduced to a zero metallic state, then, metal atoms subsequently starts to collapse with other atoms or atomic clusters to form irreparable nuclei (size 0.1–1 nm) that provide seeds for subsequent growth of particles [17]. To prevent further agglomeration, it is essential to add protective agents in order to control particle nano size [18]. Electrostatic stabilization is mainly dependent on Coulombian repulsion forces (Coulomb interaction) between the suspended nanoparticles, where ligands are adsorbed on the surface of cluster [19]. On the other hand, steric stabilization is proceeded via the coordination of organic molecules (e.g., polymers, surfactants, long-chain alcohols) on the particle surface [20]. With appropriate selections for concentration of precursors, reducers and stabilizers, highly stable/mono-dispersed nanoparticles can be produced. In this manner, preparation of polymer-stabilized nanoparticles by a chemical method involves two processes: (i) reducing metal ions to zero-valent atoms and (ii) coordination of organic molecules to nanometal. In practice, reduction can be performed via the interaction between metal ions and polymeric building blocks [14,21]. If the reduction proceeded, the structural properties of the metal nanoparticles are determined by the reduction conditions only, and the bond strength between polymer and metal nanoparticles mainly effects on the size and geometrical shape of metal nanoparticles.
Common reagents reported for synthesis of different noble metal nanoparticlesand control their growth were thiols [22], phosphines [23] phenantroline [24], and chiral diphosphite [25] are also used for synthesis of nanopalladium (PdNPs), which, in turn, can be obtained by reduction of the corresponding metal salt and encapsulated within reverse micelle [26], dendrimer [27] or biomolecules [28].
Polysaccharides were interestingly exploited for synthesis of numerous nanostructures owing to its speciality in biocompatibility, biodegradability, non-toxicity, and renewability [29,30]. In accordance to literature, polysaccharides are characterized by their excellence potentiality to perform as reducer and stabilizer in chemical reduction route for green synthesis of different metal nanoparticles, to provide an alternative route for avoiding the hazardous environmental impacts resulted from the traditional chemical and physical synthetic techniques [31,32]. Polysaccharides as a natural polymers could be produced from microorganisms, algae, plants and animals [[33], [34], [35]]. Polysaccharides could be categorized to three classes according to their ionic character; cationic (like: chitosan.etc.), anionic (like: alginate, carrageenan, arabic gum, heparin, Hyaluronan … etc …), and neutral (like: starch, dextran, agarose, pullulan ….etc …) [36,37]. Also, polysaccharides are advantageous with various properties and chemical functionality, owing to their main functional groups in their constructions.
According to literature, different metal nanoparticles were usually synthesized via chemical reduction methodology that proceeds via reduction of metal ions to generate the required nanoparticles, in the presence of an appropriate stabilizing agent to protect the nanoparticles from agglomeration. Owing to the high accessibility of polysaccharides for coordination with metal ions, they can superiorly act as anti-coagulating agents (i.e. stabilizer). Polysaccharide-metal ion complexing can subsequently be reduced, to generate small-sized/well dispersed metal nanoparticles. Once the reduction reaction is proceeded, the immobilization of nanoparticles within polymeric matrix acts in preventing their coagulation. Different polysaccharides that were reported to be functionalized as reducing and/or anti-coagulant agents for the synthesis of metal nanoparticles [[38], [39], [40], [41], [42]]. Additionally, for their exploitation to form a complex with metal ions, polysaccharides might also be advantageous with reducible characters and thus can play the role of reducer and stabilizer in the preparation of metal nanoparticles. This main role of polysaccharide can be beneficial in green production of nanostructures since it decreases the requirement for exploiting of toxic chemical reagents. Also, numerous metal nanoparticles as nano-catalysts that prepared by polysaccharides were reported in various researches, as dextrin was applied for synthesis of gold nanoparticles (AuNPs) with size average of 8.4–12.0 nm to be functional for acceleration of liquid phase oxidation of ethylene glycol [43].
Heparin & hyaluronan [29,44,45] and chitosan [46,47] and carboxylmethyl chitosan were exploited for successive synthesis of silver and gold nanoparticles. According to the reported results, gold and silver nanoparticlesthat were produced using exhibited similar sizes than that prepared using chitosan [47]. Carboxylmethyl chitosan was mentioned to have higher metal chelating power than chitosan, which was reflected in production of more coagulated nanoparticles using carboxylmethyl chitosan. Exploiting of heparin to act as reducing/anti-coagulant agent was studied to control the mean size of gold nanoparticles, while, particle size was diminished by increasing heparin concentration [44]. Gold and silver nanoparticles produced using hyaluronan was characterized by bigger size than that synthesized by heparin [45]. The produced heparin based metal nanoparticles were characterized by in vivo and in vitro anti-inflammatory characters and anticoagulant affinity [29]. In another approach, spherical AuNPs were synthesized using carboxymethyl cellulosewith size of 8.8–14.0 nm, while, lower concentration of CMC resulted in generation of heterogeneous AgNPs in nano-prism shape with dimensions of 32.0 and 92.0 nm, while nano-hexagon was synthesized with much smaller size of 17.6 nm [21].
In other researching reports, starch was capable for synthesis of spherical Cu(NO3)2 with size average of 5.0–12.0 nm to exhibit anti-bacterial activities against both of E. coli, S. aureus & Salmonella typhi [48]. Hyaluronic acid was investigated for their ability to play the dual role of reducer and stabilizer in synthesis of spherical SeNPs with particle size of 66.8 nm, to exhibit anticancer affinity for Heps solid tumor (in vivo) [49]. Alginate was used for synthesis of BiNPs with size average of 5.0–8.0 nm and porous structure, to be applicable as nano-catalyst for 4-nitrophenol reduction reaction [50]. Pullulan was used for synthesis of oval-shaped AgNPs with size average of 2.0–30.0 nm to exhibit biocidal potency against Aspergillus spp., Penicillum spp., L. monocytogenes, P. aeruginosa, E. coli, and K. pneumoniae [51]. However, Zahran et al., reported that, alginate could be functionalized for immediate synthesis of silver nanoparticles (AgNPs) under alkaline conditions with mean size of 1–4 nm to be consequently loaded on cotton fabrics for exploitation in preparation of biocidal textiles [52,53]. Hydroxy-propyl cellulose also played the role of reducing and stabilizing agent for production of spherical AgNPs with size average of 25.0–55.0 nm to be characterized by anti-microbial activity against P. aeruginosa, B. subtilis, S. aureus, E. coli, Actinomycetes, A. niger and S. epidermidis [54]. Using xylan, spherical AgNPs with size distribution of 20.0–35.0 nm were prepared to be applicable for detection of Hg2+ [55]. Exploitation of chitin was also reported for synthesis of AgNPs with size average of 5.5–15.2 nm and with mesoporous and fibrous shape to be functionalized for acceleration of p-nitro phenol reduction [56].
3. Nanopalladium (PdNPs)
Palladium belongs to group 10 in the periodic table, but the configuration of its outermost electron shells (Pd 4 d10 5s0) typical to the other members of the group (Ni 3d84s2; Pt 5 d9 6s1, and Ds 6 d9 7s1 or 6 d8 7s2). Hence, the totally empty O-shell in palladium electron configuration is a unique phenomenon that takes its properties out of that of 10 group elements. It is the least dense and it has the lowest melting point out of all platinum group metals (Ru; Rh; Pd; Os; Ir; Pt). Palladium melts slowly in concentrated nitric acid, in hot or concentrated sulfuric acid and in hydrochloric acid [17].
Common oxidation states of palladium are 0, 1, 2, and 4, though 3 and 6 values have already been reported [57,58]. There are comparatively few known compounds with palladium unequivocally in the oxidation state. Although such compounds are suggested as a mediator in many palladium-catalyzed cross-coupling reactions. But, the high cost of palladium naturally restricts its application, However nanopalladium or their alloy groups have been extensively examined. Palladium forms a versatile catalyst (Fig. 2) and speeds up petroleum crushing, selective low oxide, and alkanes oxidation [59], hydrogenation of alkynes to alkenes without further reduction into alkanes (called Lindlar's Palladium), number of carbon-carbon bond forming reactions (Heck reaction [60] and Suzuki coupling [61]), and carbon-fluoride bond formation.
 Fig. 2. Applications of PdNPs as catalyst.
Fig. 2. Applications of PdNPs as catalyst.Recently, delving into the synthesis of metal nanoparticles viz. Silver, gold, and platinum, zinc, palladium, etc. It has characterized by a great interest in the scope of physical and biological sciences [[62], [63], [64], [65]]. Among other metal NPs, Pd is gaining special signal because of its radical applications as a catalyst by a large number of organic transformation which include several types of carbon-carbon cross-coupling [66], oxidation [67] and reduction reactions [68]. Conventionally, PdNPs synthesis via different physical or chemical methods using toxic and dangerous stabilizing and reducing agents were reported, but, over the decades, In pursuit of “preserving the environment”, there's a pattern moving around bio-inspired strategies for the synthesis of metal nanoparticles [69].
Reetz et al., in 1995 represented a novel technique for synthesis of Pd nanocomposites, as Pd nanoclusters/poly(N-acetyl aniline) nano-rods were electro-deposited onto a glass carbon electrode by cyclic voltammetry (CV) [70]. They investigated that, ball-shaped nanopalladium was grown at the ends of nano-rods, forming a novel nanocomposite. The preliminary study also showed that the electrode modified with this nanocomposite matrix has high electro-catalytic activity toward 4-e'- oxygen reduction. Dhas et al., in 1998 reported that, the quasione-dimensional nanopalladium can be performed by the metallization of the deoxyribonucleic acid [71].When, the surface of this long molecule was activated with palladium ions and then immersed in a reduction solution consisting of sodium citrate, lactic acid, and dimethylamine borane. The separated metal nanoclusters gradually aggregated into a quasi1-D metallic structure with 5 nm in diameter. In addition to the nucleation of nanopalladium, the metallic deoxyribonucleic acid in the solution may be immobilized by way of alignment on a substrate by controlling the solvent evaporation.
Another interesting study for synthesis of Pd nanowire electrode by electrode position with different reducing potentials and sedimentation times was studied by Teranishi et al., in 1999 [72]. The nanowires grew under the effect produced by the suitable adsorption of sulfuric acid anion on Pd (111) plane. They mentioned that, ass the potential increased, dendritic strands with nanowires a single crystal structure became longer in terms of their length and thinner in terms of their thickness. Yonezawa et al., in 2001 suggested that, the stabilization of nanopalladium was manily dependent on the chemical bonding with nitrogen-containing organic compounds such as, phenanthroline, sodium sulfanilate, or isocyanide [73]. Moreover, Quiros et al., in 2002, hypothesized that, when the long chain of ligands increased the amount of stabilizer used and lengthens the reaction time in order to produce smaller sized nanopalladium [74].
Synthesis of n-octadecayl mercaptan (C18H37SH) protected nanopalladium at room temperature was proposed by Choo et al., as the main size was evaluated to be around 2 nm nanopalladium [75] in the presence of hexadecylamine or polyphosphine ligand, as they affirmed that, the particle size of palladium depended on the nucleation and crystal growth rate in a certain chemical environment. The study of Lu et al., 2002 [76], showed that, some ligands might be used as homogeneous or heterogeneous seeds for the subsequent growth of palladium. The seeding growth might be scaled up to produce larger and monodispersed particles, despite their structure, Therefore, the properties will be correlated to the nature of seeds. Later, amines coated the surface of nanopalladium for protection against inter-particle agglomeration resulted in a particle size of 5 nm was investigated by Ramirez et al. [77].
Jiang et al., in 2008 affirmed that, the electrochemical method is widely used for preparation of bimetallic colloids based on palladium [78]. So, the colloid palladium alloys can be synthesized if two surficial anodes are used simultaneously. As it is been taken in account the mutual reduction/oxidation between two different nanoparticles inside the bimetallic core and hereby provides a lot of choices in the architecture of metal colloid. In addition to, Yagi et al., in 2008 suggested that the formation of a self-assembly by a long-chain stabilizer occurred faster than that of a shorter one, due to the stronger hydrophobic interaction. However, the use of long-chained ligands might limit the size of nanopalladium (50 nm).
On the other hand, other researching approaches also reported that, poly(N-vinyl-2-pyrrolidone) could be widely used as a protective agent to obtain nanopalladium [79]. This soluble polymer prevented the pre-formed particles from coalescing through the vacuum effect induced by custom ring sections of polymers dispersed in the solvent. However, the higher the molecular weight of the polymer is, the larger the size of nanopalladium, and higher the thickness of the adsorbed polymer layer. Additionally, nanopalladium was successively produced by reduction of palladium acetate by octylamine in hexadecanediol [80].
More recent report of Baumann et al., in 2014 studied the examination for a series of C–H bond functionalization reactions of hetero-arenes, such as purinederivatives, benzthiazole, benzoxazole, benzimidazole and indole, mediated by Pd(OAc)2, as well-known catalyst, applicable for C–H bond functionalization. The reported study revealed that, nanopalladium with size distribution of 2–6.5 nm was rapidly grown within the reaction medium. Moreover, they mentioned that, nanopalladium could be ex-situ characterized after entrapping within a polymeric matrix such as polyvinylpyrrolidinone (PVP). The results showed that, the synthesized nanopalladium/PVP was exhibited by catalytic activities commensurate with Pd(OAc)2 in C–H bond functionalization reactions. Additionally, by monitoring the effect of reaction variables on the catalytic efficiency of the generated nanopalladium within the reaction liquor, by using polar solvents like DMF, DMSO and acetic acid, palladium salt concentration was the key variable, which could significantly effect on the catalytic potentiality of nanopalladium [81].
Also during 2014, Bi et al., presented a report for synthesis of nanopalladium that exhibited different sizes within arrange of 100–240 nm, as nanopalladium was in situ synthesized via the reduction of PdCl2 with NaBH4 as reducing agent and using of poly(methacrylic acid) (PMAA) microspheres as a stabilizing agent. The catalytic potentiality of the PMAA/Pd composite was investigated in acceleration of the reduction reaction of p-nitro-phenol. The results showed that, the reaction rate increased with elevation of reaction temperature, raising of p-nitro-phenol concentration, and uploading of nanopalladium on PMAA microsphere. The PMAA/Pd composite exhibited good stability that affirmed the protective ability of PMAA microsphere for stabilizing of nanopalladium [82].
4. Green synthesis of nanopalladium
For preparation of nanopalladium, various green synthetic techniques were reported in literature and summarized in Table 1. Considering with the exploitation of polysaccharides as synthesizers for nanopalladium, spherical nanopalladium was prepared using starch with particle size of 1.5–4.5 nm to be applicable as nano-catalyst for Suzuki reaction, Heck reaction and Sonogashira reaction [83]. While, Liu et al. were ascribed the synthetic methodology of nanopalladium with size of 3.4 nm using carboxymethyl cellulose (CMC) as a capping agent, as green and cost effective method [84]. So, it could be affirmed that, particle size, geometrical and topographical features are mainly dependent on the chemical composition of the exploited polysaccharide and the experimental conditions for generation of the as-required nanoparticles.
Table 1. Different green synthesizers for nanopalladium.
| Nano-synthesizer | Size (nm) | Shape | Reference | Reference |
|---|---|---|---|---|
| Anogeissus latifolia Gum | 4.8 | Spherical | [39] | [157] |
| Cinnamom zeylanicum Bark | 15–20 | Crystalline | [23] | [158] |
| Cinnamomum camphora Leaves | 3.2–6 | –[ | [86] | [159] |
| Curcuma longa Tuber | 10–15 | Spherical | [26] | [160] |
| Euphorbia granulate Leaves | 25–35 | – | [44] | [161] |
| Gardenia jasminoides Leaves | 3–5 | – | [27] | [162] |
| Glycine max Leaves | 15 | Spherical | [34] | [9] |
| Moringa oleifera Waste petal | 10–50 | Spherical | [42] | [163] |
| Moringa oleifera Peel extract | 27 ± 2 | Spherical | [43] | [164] |
| Pulicaria glutinosa Whole plant | 20–25 | Crystalline and spherical | [107] | [69] |
| Pinus resinosa Bark | 16–20 | Crystalline | [58] | [165] |
| Starch | 1.5–4.5 | Spherical | [62] | [83] |
| Carboxymethyl cellulose | 3.4 | [63] | [84] | |
| Acacia | 5.3 | Spherical | [16] | [16] |
| Pectin | 27.9 | Spherical | [16] | [16] |
| Chitin | 38.5 | – | [166] | |
| Agar | 12.8 | Spherical | [13] | [167] |
| Lignin | 4.5 | Spherical | [14] | [14] |
| Dextran | 17.1 | Spherical | [15] | [15] |
Momeni and Nabipour demonstrated a new technique for synthesis of nanopalladium using plant extracts of marine alga and sargassum bovinum. Water-soluble compounds generated from the marine alga extract were explained to be the reductant for the palladium ions to produce nanopalladium. The main properties of nanopalladium prepared by this method were confirmed, while, nanopalladium were exhibited by a monodispersed and octahedral geometry, with size ranged in 5–10 nm. The catalytic potency of the green synthesized nanopalladium was investigated for acceleration of hydrogen peroxide electrochemical reduction. nanopalladium modified carbon ionic liquid electrode (nanopalladium/CILE) was also reported to act as non-enzymatic sensor for determining of hydrogen peroxide. The amperometric detection ascribed that nanopalladium/CILE was a superior sensor for estimation of hydrogen peroxide in ranging of 5.0 μM–15.0 mM with a sensitivity of 284.35 mAmM−1 cm−2 and limit of 1.0 μM [85].
In 2017, Rahmati et al., reported a study for a synthetic technique to prepare nanopalladium with size distribution of 3–6 nm and was stabilized by a mixture of natural carbohydrate polymers, arabic gum and pectin, to act as bio‐organometallic catalyst. The prepared nanopalladium was applied as nano-catalyst and showed to exhibit high efficiency in acceleration of Mizoroki–Heck cross‐coupling reactions between various aryl halides and n‐butyl acrylate under solvent‐free conditions. The prepared nano-catalyst can be easily recovered from the reaction system and re-used for several times with high quantum yields [86]. On the other hand, Dinesh et al. investigated another ecofriendly synthetic methodology for preparation of nanopalladium that was achieved using Phyllanthus emblica (P. emblica) seeds as reducer. The data indicated that biogenic synthesized nanopalladium exhibited a sphere geometry with size of 28 ± 2 nm, moderate in stability. The prepared nanopalladium and the extract was evaluated for its antibacterial action by agar well diffusion method. Seed extract leaded to 8.9 ± 1.46 mm against B. subtilis, while, nanopalladium exhibited 9.6 ± 1.10 mm against S. aureus. The prepared nanopalladium and extract were examined for hemolysis that, leaded to 20% and 10% respectively. Toxicity results were evaluated against Artemia salina (A. salina). The LC50 value of green prepared P. emblica protected nanopalladium and P. emblica, while, seed extract exhibited less toxicity for A. salina in a value of 1.00 μg/mL and 1.25 μg/mL. Moreover, the prepared samples were tested for in-vitro cytotoxicity on HeLa cell lines [87].
Rakhi et al., also in 2017 investigated a simple green chemical methodology for the one-step preparation of nanopalladium using the leaf extract of Chrysophyllum Cainito in aqueous medium that acted as a source of reducing species. The preparation of nanopalladium was proceeded within 2–3 h at room temperature, whereas, at elevated temperature (70–80 °C), the production of colloidal nanopalladium was immediately completed. The prepared nanopalladium was utilized as an ecofriendly nano-catalyst for C–C coupling reactions under aerobic and phosphine-free conditions in aqueous environment. Additionally, the produced nanopalladium was also utilized as green nano-catalyst for sodium borohydride reduction of 3- and 4-nitrophenols. The produced nanopalladium approved to retain their catalytic potency for several months [88].
Doherty et al., also investigated that, phosphino-decorated polymer immobilized within ionic liquid phase protected nanopalladium (nanopalladium@PPh2-PIILP) and their PEGylated counterparts (nanopalladium@PPh2-PEGPIILP). Nanopalladium@PPh2-PEGPIILP as nanopalladium-based system with size average of 1–3 nm, was functionalized as a significantly active catalyst for hydrogenation of α,β-unsaturated aldehydes, ketones, nitriles and esters in aqueous environment, while, under mild conditions, nanopalladium@PPh2-PEGPIILP showed the highest catalytic efficiency to exhibit complete conversion with 100% selectivity for reducing the CvC bond [89]. Later, Samsonu et al., represented a study for synthesis of palladium/sucrose (Pd/S) nanoparticles in ethanol at room temperature, as a green catalyst for efficient chemo-selective reducing of aromatic nitro compounds. The reductions are advantageously performed with the aid of different accessible groups such as halo, cyanide, carbonyl, and alkoxy groups.etc [90].
One of the most recent studies was that presented by Pei et al., in 2021, in which, chitin was exploited for synthesis of nanopalladium that were expressed as new substrate/highly applicable for reduction of the environmental pollution resulted from non-biodegradable plastics. Whereas, three chitin@Pd catalysts differing in their geometrical structures, were prepared through diverse activation methodology. nanopalladium wit size distribution of 1.6–3.2 nm, were reported to be well-dispersed within microspheres, which was attributed to the interconnected nano-fibrous networks and accessible groups of chitin, while, this was affirmed by different physicochemical specifications. Pd/chitin-Ar was reported to be the best candidate of the synthesized catalysts in acceleration of CO oxidation reaction as it achieved 100% CO conversion, and was exhibited by excellent stability within 24-h. Additionally, the prepared catalyst was exploited in catalyzing the Heck coupling reaction, and displayed competitive catalytic action and stability of nearly 6 runs, with conversion of 94%. The authors affirmed that, the utilization of chitin as a type of biomasses could be superiorly applied for synthesis of catalytic constructions that would be importantly considered for the sustainable chemistry [91].
5. Nanostructures in wastewater treatement
The functionalization of nanostructures in water treatment was shown high potency, however, its expensiveness must be managed in order to compete the conventional techniques in marketing [92]. Attributing to high surface/volume ratio, nano-materials are characterized with size related properties such as higher adsorption capacity, reactivity and dissolution activities can be applicable in treatment of wastewater. Additionally, some specialized properties like quantum confinement effect, semiconduction, and superparamagnetism also resulted in their high accessibility in treatment processing. There are different recent investigations in synthesis of different nano-objects like, molecularly imprinted polymers, catalytic nanomembranes, biomimetic membrane, bioactive nanoparticles, nanosorbents and nanocatalysts for removal of toxins, microbes causing disease, organic and inorganic pollutants from water.
5.1. Adsorption
Sorbents were widely applied in purification of wastewater and tertiary treatment step for removal of the organic and inorganic pollutants. The effectiveness of the conventional sorbents that are applied in the adsorption processings like the activated carbon, ion-exchange resins.etc... are disadvantageous with lacking of high accessible active sites on the surface area, no selectivity and specificity, and without adsorption kinetics. The exploitation of nanostructures (nanosorbents) might have advantages for overcoming the challenges of conventional sorbents, owing to larger surface area of nanostructures, higher selectivity/specificity, and interchangeable pore sizes. In addition to unique structures and electronic characters of some nanostructures could lead them to be described as superior adsorbents. Table 2 represents different nanosorbents in wastewater treatment.
Table 2. Different nano-sorbents in wastewater treatment.
| Nano-sorbent | Waste type | References | References |
|---|---|---|---|
| Carbon-based nano-sorbents | Nickel ions | [162] | [168] |
| Graphite Oxide | Dyes | [163] | [169] |
| Regenerable polymeric Nano sorbents | Organic and inorganic pollutants | [164] | [170] |
| Nano-clay | Hydrocarbons and dyes | [165] | [171] |
| Nano-metal oxides | Different Heavy metals | [166] | [172] |
| Nano-Aerogels | Uranium | [167] | [173] |
| Nano-iron oxides | Hormones and toxic pharmaceuticals | [168] | [174] |
| Polymer Fibers | Arsenic and other toxic metals | [169] | [175] |
Nanostructures of metals or metal oxides including the nanosilver [93], ferric oxides nanoparticles [94], titanium oxide nanostructures [95], magnesium oxide nanoparticles [96], nano-copper oxide [97], cerium oxide nanostructures [98].etc., all these could provide a high surface area and a specific affinities for removal of heavy metals in treatment of wastewater. Additionally, common metallic oxides possess few environmental impacts owing to the low solubilities; they could be adopted as sorbents for the removal of heavy metal ions.
Hristovski et al. (2007) affirmed the superiority of the aggregated nanometal oxide media/packed in bed columns for removing of arsenate [99]. According to the batch experiments that were conducted with 16 commercial nanopowdersin water, titanium dioxide, ferric oxide, zirconium dioxide, and nickel oxide nanopowders were selected depending on the parameters that were fitted to Freundlich adsorption isotherm, for exhibiting the highest removal of arsenate in water. Studies of Singh et al. (2011) showed that different toxic metal ions, such as cobalt, nickel, copper, cadmium, lead, mercury, and arsenate could be removed from wastewater through a porous nano-zinc oxide assemblies [100]. It was approved that, ions of mercury, lead, and arsenate have stronger affinity towards nano-zinc assemblies attributing to their high electronegativity and, therefore, they exhibited better affinity for removal (100% As3+, 100% Pb2+ & 63.5% Hg2+). In recent years, there were several approaches on magnetic oxide, especially ferric oxide, being applied as nano-adsorbing reagents for purification of wastewater from different toxic metal ions, such as ions of nickel, chromium, copper, cadmium, cobalt, mercury, lead, and arsenate [94,[100], [101], [102], [103], [104], [105]]. Shen et al. (2009) were observed that the efficiency of adsorption ions of nickel, copper, cadmium, and chromium by ferric oxide nanoparticles was mainly depending on the incubation time, pH, temperature, and the amounts of the adsorbent [105].
5.2. Photocatalysis in treatment of wastewater
Photo-catalysts activated by ultraviolet radiation could oxidize the organic wastes into non-toxic compounds, such as CO2, H2O and could act also in disinfection of certain bacterial strains. Photo-Catalysis could be identified as accelerator of the photo-reaction under the effect of catalyst. Whereas, catalyst could not be changed or consumed within the reaction. In the catalyzed photolytic action, irradiation could be absorbed by adsorbed substrates. In photo-generated catalysis the photo-catalytic activities depend on the affinity of the catalysts for creation of the electron-hole pairs, that generate hydroxyl radicals, could be abled for undergoing secondary reactions. It is successfully applicable for the pretreatment of water to remove the toxic and non-biodegradable pollutants. Photocatalysis could also be exploited as a polishing step for treatment of recalcitrant organic substrates. The main barrier for its wide applicability is the slow kinetics attributing to the limited light influence and photocatalytic activities. Silica, titanium dioxide, zinc oxide, tungestun oxide, cadmium sulphide, zinc sulphide, stronchium/titanium oxide, tin oxide, ferric oxide.etc... could be exploited as photocatalysts.
5.3. Nanomembranes in treatment of wastewater
Traditional methods for wastewater treatment that include physical techniques of separation for wastes removal; biological and chemical treatment processings for the removal of dispersed solids, organic compounds and the dissolved polluting compounds or toxic reagents; and evaporation techniques and other mechanical and physical methodologies [106]. Membrane separationcould replace such mentioned techniques via the application of selectively permeable barrier suitable for permission of passing the water flow, but fine enough for retaintion of various dissolved pollutants, depending on their nature [107,108]. Membrane filtration processings were categorized depending on the membrane pore size and the particle size of the pollutants that could be retained. On the other hand, the affinity of different membranes could be enhanced; for example by improvement the flux or by decrement of the membrane fouling [109]. Characteristics of membrane that could be enhanced by the application of nanostructures are: antifouling and microbicidal characters; increment of selectivity; increment of flux (via increment of the hydrophobicity); or pore size controlling by nano-gels (shrinkage/growth by changing of pH or temperature) [110]. The applications of nanostructures in the composition of membranes could thus diminish the energy demanding, the application of chemical reagents for membrane cleaning, and cost effectiveness.
For addressing this challenging, membranes could be applicable with nanostructures to enhance the performance of membrane. This methodology is rather novel, so it is not surprisible that different items are applied to ascribe this technology; nanoparticle-improved, nano-activated, nanoparticles-based, and nano-functionalized membrane.etc. [111]. The terminology should be standardized for avoiding any ambiguity. ‘Nano-enhanced membrane (NEM)’ as ‘enhancement’ is the best description for the actinon of the nanostructures impregnated within membrane matrix [112]. Recent approaches on nanotechnology/membrane technology were focused on the synthesis of nanoparticles immobilized within the polymeric or inorganic membrane for multifunctional purposes [113]. Nanostructures are mainly applied for multifunctional purposes including nanoparticles of metal oxides, like, alamunium oxide, titanium dioxide, zeolite, and microbicidal nanostructures (for example, nano-silver and carbon nanotubes), and photocatalytic nanostructures (for example, bi-metallic nanostructures, titanium dioxide, zinc oxide) [114,115]. Application of metal oxide nanostructures in NEM for resisting the membrane from fouling and increment of the water permeability, is attributing to the hydrophilic nature of metal oxide nanostructures [116]. Recently, photocatalytic membranes were attracted the attention due to its simultaneous functionalization in the photocatalytic decomposition of the organic matters and the continuous discharging of the clean water without losing of the nano-photocatalyst [117]. Photocatalytic membranes could be synthesized by using various nanostructures through different synthetic methodologies.
TiO2 supported on polymer and metallic membranes [118,119] resulted in investigation of TiO2/Al2O3 composite membrane [[120], [121], [122], [123]], and TiO2 entrapped within doted polymeric membrane [[124], [125], [126]] that could be applicable for treatment of wastewater. Additionally, organic and inorganic ceramic membranes containing TiO2 were investigated [118,[127], [128], [129], [130]]. In accordance with most studied approaches, photocatalytic membrane might encounter different problems in techniques such as membrane structural decomposition, low photocatalytic activities and losing of uploaded TiO2 layer by time. Metallic/bi-metallic catalytic nanostructures like nano zero-valent iron (nZVI) and noble metals uploaded on nZVI were immobilized within polymeric membrane for reductive decomposition of pollutants, especially chlorinated contaminants [129,130]. nZVI acts as electron donating cite and the noble metals could catalyze the chemical reactions.
5.4. Microbicidal potency
Microbial pollution of water causes a serious threating to human health. With the emergence of resistant microbes for different microbicidal reagents [131], there is increased requirement for enhanced disinfection methodologies. Recent advances in nanobiotechnology, for the preparation of nanomaterials exhibited with specific shape and size, to be exploitable as bactericidal reagents for wastewater treatment. Diminishing the particle size was reported to have an efficient and reliable effects for enhanced the biocompatibility of the nano-objects [132,133]. The functionality of nanostructures was found to be significantly influenced by the particle size. However, few reports were considered with the effect of size diminishing on the biocidal mechanism of action for nanomaterials. Bactericidal activities were found to be related to the materials that are locally killing the bacteria or slowing down their ingrowth, without exhibiting any toxic effects to the surrounding tissues.
5.5. Nanopalladium in wastewater treatment
Among the different transition metallic nanoparticles, nanopalladium had extensively attracted the attention of researchers interested in the field of nanotechnology owing to its superior applicability in various purposes, therefore, in the following sections, the applications of nanopalladium in different purposes will be briefly illustrated; Precious metallic structures with superior catalytic action, like nanopalladium, were received considerable attention (Table 3) [[134], [135], [136]]. But, nanopalladium with controllable small sized particles and high surface energy are reported to be unstabilized and forms agglomerated masses, leading to decrease their catalytic efficiency. One of the effective methods to prevent nanopalladium coagulation is to upload such particles on supporting template [[137], [138], [139], [140], [141]]. In recent years, different palladium-based nanocatalysts were reported to be exploitable in wastewater treatment. Lebaschi et al. prepared Pd@black tea nanocatalysts that exihibted excellent catalytic performance to be applicable as environmental friendly nanocatalyst for wastewater treatment [142]. Wu et al. prepared cellulose nanocrystals-supported nanopalladium composites that exhibited high catalytic performance in reduction of methylene blue and 4-nitrophenol [143]. Chen et al. synthesized bimetallic gold/nanopalladium/graphene nanosheets to be applicable as catalyst for reduction of organic pollutants [144]. Le et al. studied the synthesis of fibrous nano-silica supported palladium nanocatalyst (Pd/KCC-1), and from the morphology of the support (KCC-1), the superior dendritic fibers resulted in poor agglomeration of nanopalladium [145]. Catalytic reduction of 4-nitrophenol and 2-nitroaniline using polyethyleneimine/polycaprolactone@ nanopalladium composite as nanocatalyst represented extremely high catalytic performance and stabilities, to be reflected in high potential applicability in water treatment [[146], [147], [148]]. Narasaiah et al., nanopalladium prepared using Pimpinella Tirupatiensis plant extract could act as promising nanocatalyst for the deterioration of organic pollutants [149]. Catalytic performance of nanopalladium for removing of rhodamine 6G (Rh-6G) dye was also approved, while, 98% of Rh-6G dye was decolorized using the prepared nanopalladium within 2 min [150].
Table 3. Applications of nanopalladium containing biopolymer in wastewater treatment.
| PdNPs/biopolymer | Application | Reaction | References |
|---|---|---|---|
| PdNPs/black tea | Organic pollutants | 4-nitrophenol | [142] |
| PdNPs/cellulose nanocrystals | Organic pollutants | 4-nitrophenol | [143] |
| PdNPs/dextran | Organic pollutants | 4-nitroaniline | [15] |
| PdNPs/cellulose nanocrystals | Dye degradation | Methylene blue | [143] |
| PdNPs/plant extract | Dye degradation | Congo red | [149] |
| PdNPs/plant extract | Dye degradation | Rhodamine 6G | [150] |
| PdNPs/lignin | Dye degradation | Reactive yellow dye 145 | [14] |
| PdNPs/acacia | Dye degradation | Reactive red 195 | [16] |
| PdNPs/pectin | Dye degradation | Reactive red 195 | [16] |
| PdNPs/chitin | Dye degradation | Acidic blue 193 | [166] |
| PdNPs/chitin | Pesticide hydrolysis | Prothiofos | [176] |
pH responsive lignin showed to be intelligent nano-engineer as reported by Ahmed et al., for synthesis of nanosilver (13.8 nm) & nanogold (5.7 nm) in acidic pH, while, nanopalladium (4.5 nm) were produced in basic pH, to be applicable for catalytic discoloration of dyes [14]. Also, Emam et al., represented a comparative overview for the capability of dextran under basic conditions in synthesis of nanosilver (3.4 nm), nanogold (8.3 nm) & nanopalladium (17.1 nm) to be exploited in catalytic reduction of para-nitro-aniline as one of aromatic pollutants [15].
Palladium and silver nanoparticles were prepared using a green synthetic technique, that were mediated with Daucus Carota leaves. Catalytic performance was also evaluated for the synthesized nanoparticles in rhodamine 6G (Rh-6G) dye removal. 98% of Rh-6G dye was catalytically decolorized with the action of exploited nanopalladium through only 2 min, whereas, the applied silver nanoparticles were taken 30 min for decolorization of 89.4% of the examined dye. This report demonstrated the catalytic effeciency green nanocatalysts for treatment of polluted water [150].
In another approach, palladium and silver nanoparticles could be prepared via the bio-mediated technique and were superiorly exploited in the degradation of the environmental pollutants. For example, the bio-mediated silver nanoparticles were superiorly exploited in azo dye removal [151,152]. Additionally, Kora & Rastogi were investigated that, non-toxic and renewable Boswellia serratamediated nanopalladium were exploited in the anthropogenic dye degradation [153]. Palladium immobilized within zinc oxide nanoparticleswere investigated to exhibit high photocatytic activities for E.coli removal from wastewater, as it was affirmed via several analytical studies, that were performed using different concentrations from palladium in zinc oxide nanoparticles [154].
Films of nanocomposites were also prepared from palladium acetate and polyetherimide, whereas, their chemical interactions via hydrogen bonding with palladium based nanoparticles were studied to approve the affinity of the prepared films in wastewater treatment. Under different conditions, through both of in-situ and ex-situ methodologies, nanopalladium were nucleated within the polymer matrix. This represented the opportunities for designing materials with tunable characters [155].
In another researching report, the particles of nanocatalyst were anchored on carbon nanotubes (CNT) that were grown on the porous carbon foams. Such structure was advantageous with the high surface-activity with the robust and recyclability structural advantages for the porous solid membranes compatible for water treatment purpose. Whereas, three types of palladium-based nano-catalytic surfaces were investigated as follows; Pd nanoparticles with a layer of oxide (PdO-coated Pd), metallic palladium (Pd), and Pd nanoparticles uploaded with thin film of silver (Ag-Pd) [156]. The catalytic performance was compared via monitoring the rate of atrazine degradation in water samples. It was investigated that all three prepared catalysts were exhibited with high affinity for atrazine degradation, whereas, palladium oxide-coated nanoparticles showing the highest catalytic performance.
Eventually, the superior potentiality of green synthesized nanopalladium ingrained from different polysaccharides (such as chitin, starch, agar, dextran, carboxymthyl cellulose, ligin, agar, acacia gum .etc.) was summarized and highlighted in Table 4. Compared to other nanostructures, nanopalladium exhibited remarkable higher removal of different organic pollutants such as, 4-nitrophenol, 4-nitroaniline, reactive yellow dye 145, reactive red 195, acidic blue 193, methylene blue, methyl red, congo red, prothiofos and methyl orange. As reported in Table 4, the rate constant of pollutants removal was extremely high in case of using nanopalladium rather than nanosilver nad nanogold for the same pollutant.
Table 4. The efficiency of nanopalladium in pollutant removal comparing with other NPs.
| Nanoparticles | Pollutant | Rate constant (K1, min−1) | References | |
|---|---|---|---|---|
| PdNPs | PdNPs/black tea | 4-nitrophenol | 35.4 × 10−3 | [142] |
| PdNPs/dextran | 4-nitroaniline | 176.6 × 10−3 | [13] | |
| PdNPs/dextran | 4-nitroaniline | 205.7 × 10−2 | [15] | |
| PdNPs/CNCs | 4-nitrophenol | 5.7 × 10−3 | [143] | |
| PdNPs/lignin | Reactive yellow dye 145 | 314.0–478.2 × 10−3 | [14] | |
| PdNPs/acacia | Reactive red 195 | 408.8 × 10−3 | [16] | |
| PdNPs/pectin | Reactive red 195 | 143.8 × 10−3 | [16] | |
| PdNPs/chitin | Acidic blue 193 | 424.9 × 10−3 | [166] | |
| PdNPs/alginate | Methylene blue | 835.5 × 10−3 | [12] | |
| PdNPs/dextran | Methyl red | 142.2 × 10−2 | [15] | |
| PdNPs/plant extract | Congo red | 128.1 × 10−3 | [149] | |
| PdNPs/chitin | Prothiofos | 11.12 × 10−5 | [176] | |
| AgNPs | AgNPs/dextran | 4-nitroaniline | 94.5 × 10−3 | [13] |
| AgNPs/dextran | 4-nitroaniline | 14.8 × 10−3 | [15] | |
| AgNPs/CMC | 4-nitroaniline | 30.2 × 10−3 | [177] | |
| AgNPs/lignin | Reactive yellow dye 145 | 37.8–51.5 × 10−3 | [14] | |
| AgNPs/alginate | Methylene blue | 62.1 × 10−3 | [12] | |
| AgNPs/agar | Methylene blue | 130.3 × 10−3 | [167] | |
| AgNPs/dextran | Methyl red | 46.8 × 10−3 | [15] | |
| AgNPs/agar | Methyl orange | 56.3 × 10−3 | [167] | |
| AgNPs/chitin | Prothiofos | 7.84 × 10−5 | [176] | |
| AuNPs | AuNPs/dextran | 4-nitroaniline | 38.5 × 10−3 | [13] |
| AuNPs/dextran | 4-nitroaniline | 174.6 × 10−3 | [15] | |
| AuNPs/lignin | Reactive yellow dye 145 | 31.7–33.6 × 10−3 | [14] | |
| AgNPs/alginate | Methylene blue | 214.4 × 10−3 | [12] | |
| AuNPs/dextran | Methyl red | 124.8 × 10−3 | [15] | |
| AuNPs/chitin | Prothiofos | 9.15 × 10−5 | [176] | |
6. Conclusion
Exploitation of nanostructures as catalysts (nano-catalysts) for removal or degradation of water pollutants has gained considerable interest. Different metallic nanoparticles were approved to be efficiently exploitable for wastewater treatment. However, nanopalladium have a great catalytic potentiality to be applicable in water treatment. To provide an alternative route for avoiding the hazardous environmental impacts resulted from the traditional chemical and physical synthetic techniques, green synthetic orutes for preparation of nanostructures were extensively considered in numerous reports. This review considered with representing green synthesis of nanostrucutres, chemistry of nanopalladium, green synthesis of nanopalladium, application of different nanostrucutres in wastewater treatment, the recent advances on green synthesis of nanopalladium, and their applications in water treatment. According to the currently represented recent reports in literature, the exploitation of nanopalladium in wastewater treatment could be ascribed as investigative/efficient pathway for more accurate and precise ways for removal of water pollutants on both of small and large scaled applications.
Compliance with ethical standards
The authors declare that they have no competing financial interest.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.