1. Introduction
The first bioactive glass (BG) was invented by Prof. Larry Hench at the University of Florida in 1969 [1]. This bioactive glass has a composition of 46.1SiO2-24.4Na2O-26.9CaO-2.6P2O5 (mol%), it was later termed 45S5 Bioglass®, and exhibits as a key property the formation of a bond with bone [1], [2]. From a compositional viewpoint, bioactive glasses can be basically divided into three groups, depending on the representative network former oxide present in the formulation, i.e., SiO2-based (silicate), B2O3-based (borate) and P2O5-based (phosphate) systems. The first group comprises a wide range of glass formulations, including 45S5 Bioglass® and other typical compositions such as 1393 BG (wt%: 53SiO2-6Na2O-12K2O-5MgO-20CaO-4P2O5) [3], [4], [5]and S53P4 BG (53%SiO2-23%Na2O-20%CaO-4%P2O5) [6], [7], [8] commercially named as BonAlive® (BonAlive Biomaterials, Turku, Finland).
Borate glasses are characterized by their higher reactivity in comparison to silica-based glasses, which results in faster bioactive kinetics [5], [9]. Phosphate glasses are resorbable materials and their dissolution rate can be tuned according to their oxide composition [10].
Various amounts of other oxides are incorporated in silicate, borate or phosphate BGs to impart particular properties to the material; for instance, CaO, K2O, Na2O and MgO are useful to adjust the surface reactivity in the biological environment; ZnO, CuO and Ag2O allow the release of biologically active ions with antibacterial properties [11].
BGs can be produced by two different methods; namely the classic oxide melting procedure and the sol-gel method. Since sol-gel BGs are more bioactive and bioresorbable [12], [13], [14] than melt-quenched glasses, in some applications the use of sol-gel BGs is preferred. The sol-gel method has advantages of compositional purity and molecular mixing, allowing control of the sample porosity by changing the processing conditions and/or by adding suitable templates [15]. The use of templates during the sol-gel process generates products with an ordered mesoporous structure which induces higher bioactivity with respect to melt-quenched glasses [16] and makes them suitable for use in drug delivery applications [17], [18], [19], [20].
Bone reconstruction surgeries can be compromised by osteomyelitis caused by bacteria infection and to treat these, systemic antibiotic administration, surgical debridement, wound drainage and implant removal are applied [21]. These methods are not always effective, so the patients may require subsequent surgeries to combat bacterial infections. Introduction of a local drug release system into the implant site can be another approach to treat the problem [22]. This treatment offers high drug delivery efficiency, continuous action, reduced toxicity and convenience to the patient [21], [23]. Mesoporous bioactive glasses (MBGs) are highly attractive for such applications.
In 2004, for the first time, Yan et al. [20] developed MBGs with the combination of sol-gel and surfactant templating methods. The obtained MBG particles were around several tens of micrometers in diameter, with highly ordered 5 nm mesopore channels. In comparison with non-mesoporous bioactive glasses (NBGs), MBGs have enhanced surface area, higher pore volume, better ability to induce in vitro apatite mineralization in simulated body fluid (SBF) and excellent cytocompatibility [24], [25], [26], [27].
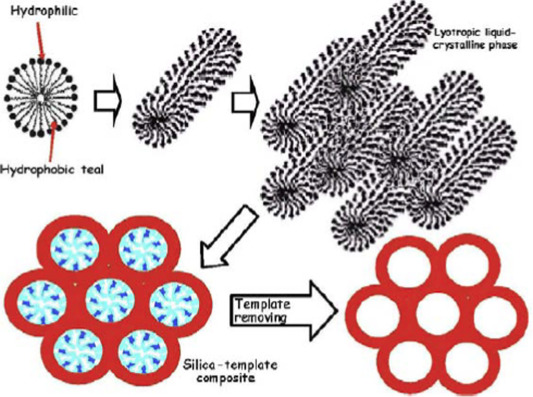
For the preparation of MBGs, the addition of structure-directing agents (e.g. Pluronic P123, F127 and cetrimonium bromide, CTAB) is essential to obtain well-ordered pore structures. Under appropriate synthesis conditions, these agents self-organize into micelles. Micelles link the hydrolysed silica precursors through the hydrophilic component and self-assemble to form an ordered mesophase [18], [28]. Then, the mixture reaction system undergoes evaporation-induced self-assembly (EISA) process. Once the mixture is dry and the surfactant has been removed, a well-ordered mesoporous structure is obtained, having high surface area and high porosity. The basic process to produce mesoporous silica is illustrated in Fig. 1 [28].

Fig. 1. Steps required to synthesize mesoporous silica from a micellar solution, according to Arcos and Vallet-Regi [28].
(Reproduced with permission of Elsevier).In 2006 and 2008 MBG powders with 58S and 77S compositions having excellent in vitro bioactivity were prepared by hydrothermal treatment using P123 [19], [29]. By using the same method, in 2008 Li et al. [30] prepared Mg, Zn or Cu containing MBG particles. Later in 2010 CaO-SiO2 MBG particles were synthesized for hemostatic applications [31].
There is a general trend to upgrade the properties of MBGs with some of the well-known therapeutic ions [11], [32], [33], [34]. By substituting small amounts of oxides, the osteogenesis, antibacterial capacity, angiogenesis or cementogenesis [34], [35], [36] effects of MBGs can be improved.
Fig. 2 (left) illustrates the year of discovery and the average time required for the in vitro bioactive response of three different silicate glass families, MPGs (melt-prepared glasses), SGGs (sol-gel bioactive glasses) and MBGs (Mesoporous bioactive glasses). In addition, the schematic diagram in Fig. 2(right) gives information about the biological function of some metallic ions that can be added to MBG compositions to enhance their biological functionalities [37].

Fig. 2. (Left): Discovery years of three bioactive silicate glass types showing the average time required for achieving bioactivity, determined by the formation of HA on the surface upon immersion in SBF. (Right): Typical metallic ions added to these glasses and their biological functions.
(Modified from ref. [37]).Prevention against bacterial infection is an essential need in orthopedic surgery due to the significant medical complications to patients related to infections [38]. Although strict hygienic protocols and preventive antibiotic prophylaxes have drastically reduced the percentage of postoperative infections, many bacterial species have developed selective resistance against antibiotics that can cause serious infections, which are hard to recover from and usually lead to second operations.
An alternative to the systemic delivery of antibiotics is the use of orthopedic devices or prostheses made from synthetic materials with intrinsic antibacterial properties [39], [40], [41], [42]. In this context, silica-based bioactive glasses offer a potential alternative to the systemic delivery of antibiotics for prevention against bacterial infections. The antibacterial properties of bioactive glasses have been investigated by Stoor et al. [43], [44], [45] from the clinical point of view. According to their study on S53P4 BG, in an aqueous environment, ions (Ca2 +, Na+, PO43 −, and Si4 +) are released from the S53P4 BG which results in a rise in pH and osmotic pressure in its vicinity. These factors potentially influence the viability of oral microorganisms at the dentogingival margin indicating potential for use in dental applications. The antibacterial effects of a paste made of a bioactive glass (S53P4) on oral microorganisms were examined [43]. It was found that Actinomyces naeslundii bacteria lost its viability within 10 min when exposed to calcium, phosphorus, silicon and sodium dissolution products. The loss of viability was 60 min for Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Streptococcus mutansbacteria. Actinobacillus actinomycetemcomitans has been suggested to play a role in juvenile periodontitis [46], [47], Porphyromonas gingivalis has been associated with destructive periodontal lesions in adults [48], [49], [50] and Streptococcus mutans is considered to play a major role in caries [51].
Indeed the antibacterial effect of BGs is used in clinical settings to combat osteomyelitis [52], [53], [54], [55], an infection of bone and bone marrow. Polymethyl methacrylate (PMMA) beads mixed with antibiotics have been used in the treatment of osteomyelitis [56]. However, after two weeks of insertion into the bone defect, these beads must be removed by surgery [57], since beads release about 24% (± 11%) of their antibiotic content (mini-beads even up to 93 ± 1.4%) [58]. Besides the antibiotic release issue, unexpected bone loss can occur when these beads are used [59].
Having discovered that S53P4 bioactive glass has antimicrobial properties [4], [7], [54], this glass took the place of polymer beads in the treatment of chronic osteomyelitis [53], [54], [60]. Polymer beads are effective against osteomyelitis treatment when they are mixed with antibiotics; however in this case they have to be removed by surgery due to their release of antibiotics and bone loss issues [58], [59]. Compared with polymer beads, bioactive glasses are osteoconductive and biocompatible materials [60]. In addition to their potential use in bone and tissue repair applications, BGs provide antibacterial and blood vessel promoting properties [11], [45].
A range of polymer based antibacterial carriers is available which could be combined with BGs for achieving improved antibacterial effects. For example, cationic proteolytic-resistant polymers with polyamide backbones have been tested for their antimicrobial activities and their cytotoxicity against primary human dermal fibroblasts [61]. These polymers were demonstrated to elicit fast bactericidal activity against A. baumanii, E. coli, K. pneumonia and P. aeruginosaand were non-toxic to epithelial cells which made them safe and potentially useable as broad spectrum ophthalmic antibiotics. Gelatin and antifungal drug-loaded gelatin fiber mats have been produced via electrospinning method [62]. Due to their cell compatibility and antifungal properties, electrospun antimicrobial nanofibers can be used in several applications, as scaffolds in controlled drug delivery, wound dressings, tissue engineering, stem cell regeneration and differentiation, and also in food packaging [63], [64], [65], [66]. In addition, gentamicin has been incorporated into a layer by layer (LbL) coating with clay interlayer barrier to achieve a controlled bactericidal effect [67], [68], [69]. However, the combination of such polymeric substrates (antibiotic carriers) with BGs to exploit potential synergistic effect of antibiotics and antibacterial ions has not been investigated.
In general terms, three approaches have been used to investigate the antibacterial activity of bioactive glasses. The first one is based on bioactive glasses that change the local physiological conditions when implanted in the body. The second one includes doping the bioactive glasses with antibacterial metallic elements, so that the glass degrades by releasing ions and induces the bactericidal effect. The last approach investigates antibiotic added to bioactive glasses [17], [34], [35], [70], [71], [72]. Moreover, mesoporous bioactive glasses, due to their ordered mesopores which can incorporate biomolecules and drugs, are suitable to achieve the synergistic release of drugs and antibacterial metallic ions to increase the effectiveness against bacteria and to impart other biological effects [73], [74], [75].
Considering the interest in combatting infections with minimal use of antibiotics [33], [38], however, it is important to investigate antibiotic-free BGs which achieve antibacterial effects exclusively by the release of metallic ions. Therefore the aim of this review is to summarize the information available in the open literature on the antibacterial properties of mesoporous silica-based bioactive glasses with emphasis on the specific antibacterial effects of therapeutic ionic dissolution products without any drugs or antibiotics added.
The studies reviewed in this article consider thus the antibacterial activity of sol-gel derived SiO2-CaO and SiO2-CaO-P2O5 mesoporous bioactive glasses based on incorporation of antibacterial metallic ions into the glass structure. The composition dependent dissolution rate and the concentration of the doped elements affect the antibacterial efficiency of bioactive glasses. Studies have shown that a balance between antibacterial activity and biocompatibility is required since a high dose of metallic ion addition can lead to cytotoxicity [76], [77], these aspects are also addressed in this review.
2. Mesoporous silica-based bioactive glasses doped with antibacterial elements
2.1. Silver doped-MBGs
Silver ion has been the most studied metal ion as a biocide (defined in the European legislation as a chemical substance or microorganism intended to destroy, deter, render harmless, or exert a controlling effect on any harmful organism by chemical or biological means), as it presents a broad and strong antimicrobial behavior [78], [79], [80]. Ag has no toxicity effect to human cells in suitably low concentrations, and it can provide bioactive glasses with extra functionality in addition to their inherent osteoconductivity and bone bonding ability [81], [82], [83], [84], [85]. As metallic silver reacts with moisture on the skin surface or with wound fluids, silver ions are released that damage bacterial RNA and DNA, hence inhibiting replication. In the case of silver, inactivation of critical enzymes of the respiratory chain (e.g. succinate dehydrogenase) by metal binding to thiol groups and induction of hydroxyl radicals appear to play a major role [86].
Silver is potentially cytotoxic if released in great amounts and thus, strict control of the introduced Ag amount is necessary. It is well-known that surface functionalization of glasses can occur by ion-exchange treatments while enabling the bulk structure and main properties to remain unaltered. In such a way silver can be introduced by ion exchange in the outer layers of glass surfaces, conferring to them antibacterial properties while maintaining the intrinsic BG characteristics [76], [77], [87].
A wide range of silver doped silica glasses (Ag-BG) have been developed by sol-gel methods [81], [82], [88], [89], [90], [91], [92], [93], [94], [95] to create bioactive glasses exhibiting inhibitory effects on bacterial growth.
Among these studies, BGs exhibiting mesoporous structure were produced via sol-gel methods [81], [82], [92] and via a structure-directing agent assisted sol-gel technique [94], [96], [97].
In this context, incorporation of Ag2O into sol-gel produced bioactive glass compositions (45S5 BG: 46.1SiO2-24.4Na2O-26.9CaO-2.6P2O5 (mol%)) has been reported to minimize the risk of microbial contamination through the leaching of Ag+ ions [81], [82].
In 2000, [81] the incorporation of 3 wt% Ag2O in 76SiO2-19CaO-5P2O5 (wt%) sol-gel produced mesoporous bioactive glass was shown to exhibit a bacteriostatic effect on Escherichia coli (E. coli) with 0.01% reduced bacterial growth without compromising the glass bioactivity. Sol-gel derived 3 wt% Ag2O incorporated mesoporous silica bioactive glass in the SiO2-CaO-P2O5 system showed good antibacterial property against E. coli, Pseudomonas aeruginosa (P. aeruginosa) and Staphylococcus aureus (S. aureus). The effect was attributed to the leaching of silver ions from the glass matrix [82]. It has been confirmed that Ag+ ions in concentrations of 0.05–0.20 mg/ml released from Ag-doped BGs inhibited the growth of different bacterial strains (E. coli, P. aeruginosa and S. aureus) [82].
In the study of Saravanapavan et al. [88], the introduction of Ag2O into sol-gel derived bioactive glass compositions was aimed at minimising the risk of microbial contamination through the potential antimicrobial activity of the leaching Ag+ ions. It was shown that the bioactive glass doped with Ag2O was bacteriostatic and elicited a rapid bactericidal reaction.
Another study reported that 60SiO2-2Ag2O-34CaO-4P2O5 (mol%) bioactive glass produced by sol-gel method was suitable to coat surgical sutures. These sutures were shown to be bioactive, and had antimicrobial and bactericidal properties against Staphylococcus epidermidis (S. epidermidis) [89], [90]. Moreover, 1 mol% Ag2O incorporated sol-gel derived 70S30C (70SiO2-30CaO) (mol%) bioactive glass scaffold was produced, which released 0.95 μg/ml of Ag+ ions in 23 h, giving a bactericidal effect on E. coli, P. aeruginosa and S. aureus cultures [91].
A study comparing the antibacterial activity of a sol-gel derived silver-doped 64SiO2-26CaO-10P2O5 (mol%) BG with its undoped counterpart against E. colispecies showed that the silver-free BG had neither bacteriostatic nor bactericidal effects; however 5 mol% Ag2O doped BG was found to have > 99% killing efficiency towards E. coli [92]. In related studies, 4 and 8 wt% Ag2O were added to 62.3SiO2–28.9CaO-8.6P2O5 (wt%) sol-gel obtained bioactive glass to investigate and to compare their antibacterial behavior with that of undoped BG against E. coli DH5α ampicillin-resistant and Streptococcus mutans (S. mutans) [95].
Both silver loadings demonstrated minimum bactericidal concentration (MBC), of 100–150 mg/ml for E. coli and 5–10 mg/ml for S. mutans, whereas the MBC for the undoped BG was > 300 mg/ml.
3 wt% Ag2O was incorporated into structure-directing agent (Pluronic P123) assisted sol-gel derived 73SiO2-13CaO-11P2O5 (wt%) mesoporous bioactive glass system to investigate the antibacterial properties of this glass [94]. Ag-MBG showed a very good anti-bacterial effect against S. aureus strain, with strong evidence of bactericidal activity at 0.5 mg/ml of glass concentration. MBC decreased from 2.2 × 109 to 2 × 104 CFU/ml from undoped to 3 wt% Ag2O doped MBG (> 99.9% killing efficiency).
58S (60SiO2-36CaO-4P2O5) (mol%) mesoporous BGs produced via structure directing agent (P123) assisted sol-gel method was doped with 1 mol% Ag2O and it was shown that this glass exhibited 100% killing efficiency against E. coli and S. aureus [98].
Table 1 presents a summary of compositions, synthesis methods and results obtained from the studies involving silver containing MBGs discussed above.
Table 1. Summary of reviewed publications on silver containing MBGs used in antibacterial applications.
| Reference | Composition | Synthesis method | Antibacterial Results |
|---|---|---|---|
| Bellantone, Coleman, and Hench 2000 [81] | 76SiO2, 19CaO, 5P2O5, 3Ag2O (wt%) | Sol-gel | Bacteriostatic effect on E. coliMG1655 with 0.01% reduced bacterial growth |
| Bellantone, Williams, and Hench 2002 [82] | 76SiO2, 19CaO, 5P2O5, 3Ag2O (wt%) | Sol-gel |
MBCs (99.9% killing) for AgBG of 1, 0.5, and 0.5 mg/ml For E. coli, P. aeruginosa, and S. aureus, respectively |
| Balamurugan et al. 2008 [92] | 64SiO2, 26CaO, 10P2O5, 5Ag2O (mol%) | Sol-gel | MBC of Ag-BG: 1 mg/ml for E. coli(> 99% killing efficiency) |
| Palza et al. 2013 [95] | 62.3SiO2, 28.9CaO, 8.6P2O5, 4 and 8Ag2O (wt%) | Sol-gel | 4Ag2O and 8Ag2O: MBC 100–150 mg/ml for E. coli and 5–10 mg/ml for S. mutans |
| Gargiulo et al. 2013 [94] | 73SiO2, 13CaO, 11P2O5, 3Ag2O (wt%) | Structure directing agent assisted sol-gel | MBC decreased from 2.2 × 109 to 2 × 104 CFU/ml for 3Ag2O doped BG (> 99.9% killing efficiency) |
| H. Zhu et al. 2014 [98] | 60SiO2, 36CaO, 4P2O5, 1Ag2O (mol%) | Structure directing agent assisted sol-gel | 100% killing efficiency towards E. coli and S. aureus |
2.2. Copper doped-MBGs
Copper is a naturally occurring element in the human body being essential to numerous metabolic processes [99], [100]. It is, in the right quantities, non-toxic to human tissues, but is known to have a strong effect on microorganisms. The antibacterial properties of CuO doped mesoporous bioactive glasses have already been proven in many studies [95], [101], [102]. Due to their controllable dissolution rate, soluble glasses represent an attractive delivery system for antibacterial copper ions.
In one of these relevant investigations, 62.3SiO2–28.9CaO-8.6P2O5 (wt%) sol-gel produced bioactive glass was doped with 4.7 and 9 wt% CuO to investigate its behavior against E. coli DH5α ampicillin-resistant and S. mutans bacteria [95]. These Cu-doped glasses had MBC of 100–150 mg/ml for E. coli and 5–10 mg/ml for S. mutans bacteria. The Cu release in SBF from 4.7 wt% CuO doped MBG was in the range 20–60 ppm and for 9 wt% doped ones, it was in the range 20–80 ppm.
A further study [101] was carried out to prepare structure directing agent assisted sol-gel derived copper-doped (molar: 0, 1, 2 and 5%) MBG scaffolds. These scaffolds were shown to possess good biocompatibility with no significant cytotoxic effect on human bone marrow stromal cells (hBMSC) and in addition the incorporation of 5 mol% Cu induced a significant antibacterial effect against E. coli. The released Cu2 + ions from MBG scaffolds significantly inhibited the viability of Escherichia coli, as shown in Fig. 3 [101].

Fig. 3. Released Cu2 + ions from MBG scaffolds significantly inhibited the viability of bacteria according to ref. [101]. ⁎0Cu-MBG group compared to blank control (p < 0.05). ⁎⁎5Cu-MBG group compared to blank control and 0Cu-MBG group (p < 0.01). The Cu-containing scaffolds significantly inhibited bacterial viability compared to scaffolds without Cu.
(Modified from ref. [101]).A glass system made by sol-gel method with the composition 75SiO2-(25-x)CaO-5P2O5-xCuO (x = 0, 2, 5 mol%) was incorporated into a nanocoating to produce nanocomposite films for wound healing applications [102]. 5 mol% CuO doped films had better antibacterial activity than pure and 2 mol% CuO doped films against E. coli, due to increased Cu ion release into DMEM medium after 7 days (0.0491 ppm vs 0.0391 ppm, respectively).
For the development of wound dressings, prevention against bacterial infections and enhancement of angiogenesis are the key issues which have to be taken into account. Wounds heal faster with the transportation of nutrients and removal of waste products from the tissues; therefore new blood vessel formation (angiogenesis) is very important [103], [104]. When a wound is infected by bacteria, the process of healing will take longer [105]; which has to be prevented or treated with appropriate biomaterials like BGs [102]. In terms of these requirements, Cu-BG nanocomposite films are promising biomaterials for wound dressing applications also providing antibacterial properties.
Composites of nanofibrillated cellulose (NFC) and Cu doped (2 and 5 mol%) MBG which have molar ratios of Si/Ca/P = 80/15/5 were developed in aerogelform [106]. After successful production of these composites, their dissolution behavior, mineralization, cytotoxicity, angiogenic performance and the antibacterial properties were examined against E. coli.
Cu-MBGs were shown to have ordered and hexagonally packed mesoporous structure, high surface area and large volume of pores. These endow Cu-MBGs with high bioactivity when the material is immersed in SBF.
When Cu-MBGs were added to NFC fibrils, they retained the absorption capability of NFC aerogels, therefore controlling the moisture around the wounds, which is beneficial to support the wound healing process.
These aerogels enhanced the gene expression of VEGF A, VEGF C, PDGF B and FGF 2 in the culture of 3T3 fibroblasts. 5% Cu doped MBG-NFC composite induced HUVEC sprouting and promoted ECM production. Both the NFC-Cu-MBG composites and Cu-MBG (2 and 5 mol% Cu) killed the E. coli strains, the effect of 5 mol% Cu being higher than that of 2 mol% Cu MBG.
In a later study, MBGs with 2% molar percentage of Cu (molar ratio Cu/Ca/Si = 2/13/85) and 5% molar percentage of Cu (molar ratio Cu/Ca/Si = 5/10/85) were prepared by a one-pot ultrasound-assisted sol-gel procedure and the final textural features, the in vitro bioactive responses and the antibacterial properties of the synthesized MBGs, were tested against E. coli, S. aureus and S. epidermidis [107].
2% mol Cu-MBG had 550 m2 g− 1 surface area, 2.6 nm size of mesopores, showing in vitro bioactive behavior and a sustained release of Cu2 + ions into SBF. When 2% mol Cu-MBG was exposed to 1 day of incubation in the three bacteria, approximately 30 to 40% of inhibition of bacteria growth was observed. For 3 days of incubation, E. coli and S. aureus were killed to an extent of 70 to 75% and S. epidermis was nearly up to 50%.
According to the final properties, this Cu-MBG which has both excellent bioactivity and antimicrobial property can be considered a suitable candidate to prevent infections and to treat bone defects.
2.3. Zinc doped-MBGs
Zinc has important effects in the development, formation and metabolism of bone cells [108], [109], [110], in the growth of blood vessels [111] and as antibacterial agent [112]. It also improves wound healing [113], [114]. Zinc provides antibacterial activity by inhibiting glycolysis, transmembrane proton translocation and acid tolerance in bacterial cells [115]. Having high impact on bone formation and growth and with its antibacterial properties, zinc ions are being increasingly considered in combination with BGs for bone regeneration applications [110].
80SiO2-15CaO-5P2O5 (mol%) mesoporous bioactive glass (MBG) scaffolds doped with 4 and 7 ZnO (mol%) were investigated for their cytocompatibility and antibacterial properties against S. aureus [116]. 4 ZnO (mol%) substitution was shown to make the MBG a suitable candidate for bone regeneration applications [116] since scaffolds with this composition led to better human osteoblast-like (HOS) cell development than the ones with 7 ZnO (mol%). This is likely due to the amount of Zn2 + ions released from the 4 mol% ZnO scaffold being more effective than the amount release from the 7 mol% ZnO scaffolds. In addition, 4 mol% ZnO scaffolds provided better antibacterial properties against S. aureus, which can also be related with the more effective Zn ion release from 4 mol% ZnO doped MBG than the 7 mol% doped one.
In a recent study [37], the inclusion of 4 ZnO (mol%) substituting SiO2 in 80SiO2-15CaO-5P2O5 (mol%) BG scaffolds was reported to drastically increase the amount of dead bacterial cells (S. aureus). Also, this amount of added ZnO increased the osteoblast development when zinc containing scaffolds were soaked in extracts of culture medium for 1, 3 and 6 days.
Atkinson et al. [117] prepared three MBGs having composition 70SiO2-(26-x)CaO-4P2O5-xZnO (x = 0, 3 and 5 mol%) by the combined sol-gel process and polymer templating methods. Their antibacterial properties were tested against Bacillus subtilis (B. subtilis) and Pseudomonas aeruginosa (P. aeruginosa). 3 mol% ZnO doped MBGs had 40% inhibition for B. subtilis and up to 31–35% inhibition for P. aeruginosa after 2 h of incubation. 5 ZnO (mol%) doped MBG showed the highest antibacterial effect with 91.3% inhibition for B. subtilis after 2 h incubation, as well as 89.4% inhibition for P. aeruginosa. The higher antibacterial inhibition achieved by the 5 mol% ZnO doped MBGs in comparison to the 3 mol% ones can be correlated with higher zinc concentration determined in the SBF solution after the 14th day of immersion. 2 ppm of zinc concentration was observed for 5 mol% ZnO MBGs and 1.8 ppm for the 3 mol% doped ones. However, the 5 mol% ZnO composition had a lower rate of hydroxyapatiteprecipitation, which is a prominent marker of bioactivity for BGs [118]. The authors suggested that the best performing material from this study for implant applications was the 3 mol% ZnO added MBG, since it displayed medium HA precipitation and antibacterial properties.
2.4. Cerium doped-MBGs
It has been reported that Ce3 + ions reduce enamel demineralization, are neuroprotective [119] and promote antibacterial behavior when added into biomaterials [120].
Goh et al. [120] added 1, 5 and 10 mol% CeO2 into 50SiO2-45CaO-5P2O5 (mol%) mesoporous silicate glasses via quick alkali mediated sol-gel method. The antibacterial properties were investigated using the quantitative viable count method. It was found that 5 and 10 mol% Ce-MBGs exhibited significant antibacterial properties compared to 1 mol% Ce and undoped samples. The antibacterial mechanism of cerium (III) ion was reported to be by binding rapidly to E. coli cells, inhibition of endogenous respiration of cells as well as penetration into the cytoplasm of the cells and interfering with their metabolic functions [121]. All samples were confirmed to have suitable bioactivity, with induced formation of apatite particles upon immersion in simulated body fluid (SBF). These Ce-MBGs were reported to have potential applications in the bone regeneration field.
2.5. Gallium doped-MBGs
The US Food and Drug Administration (FDA) has approved Ga3 + as an effective agent for treating bone resorption, autoimmune disease, for inhibition of biofilm formation, for bone, colon and prostate cancer treatments [122] and also for fighting against both Gram-positive and Gram-negative bacteria [37], [123], [124], [125], [126], [127], [128].
Salinas and Vallet-Regí [37] carried out a study on the production of scaffolds made of mesoporous 3.5 Ga2O3 (mol%) substituted for SiO2 in 80SiO2-15CaO-5P2O5 (mol%) bioactive glasses. Ga3 + ions were hardly released from the glass network; therefore it is unlikely that they imparted antibacterial property to the glasses.
A recent study done by Pourshahrestani et al. [126] investigated the effects of 1–3 Ga2O3 (mol%) addition on the antibacterial properties of 80SiO2-15CaO-5P2O5 (mol%) ordered mesoporous bioactive glasses, in which they found that 3 Ga2O3 (mol%) addition had the highest antibacterial rate against S. aureus with 99% after 12 h of incubation. The highest antibacterial rate can be related with the highest Ga ion concentration after the release into Tris–HCl solutions. After 150 h of incubation, solutions of 1, 2 and 3 mol% Ga2O3 doped MBGs had around 0.1, 0.23 and 0.32 ppm of Ga ion concentrations, respectively.
Moreover, Sanchez-Salcedo et al. [127] synthesized mesoporous glasses with the composition 80SiO2-15CaO-5P2O5 (mol%), containing 5 mol% of Ga2O3 via evaporation induced self-assembly method. It was shown that the Ga3 +concentrations released from MBG into Dulbecco's Modified Eagle Medium (DMEM) and Todd Hewitt Broth (THB) were in the non-cytotoxic levels, also in the effective antibacterial range against P. aeruginosa and not far from the effective range against S. aureus. In DMEM, the maximum Ga3 + concentration obtained was 2.5 ppm, which is below the toxicity limit of Ga3 + in blood plasma (14 ppm) [38]. In THB the release of Ga3 + concentration was 9.8 ppm, which is 140 times higher than the IC90 (drug concentration inhibiting 90% of parasite's activity) of P. aeruginosa and 2 times lower than that of S. aureus [117]. Therefore, this MBG was considered a promising material for bone regeneration applications.
3. Summary and outlook
BGs are being used in bone regeneration applications as fillers in composites [129], as scaffolds for bone tissue engineering [72], [77], [130] and as coatings of implants [131]. The failure of implants may happen due to bacterial colonization or infections [38]. These failures are mostly solved by the administration of systemic antibiotics to the patient. This solution can lead to allergic reactions, microbial flora depletion and bacterial resistance.
Due to these limitations of antibiotics, the modification of biomaterials with alternative antibacterial agents [132] is being intensively investigated. In this perspective, bioactive and soluble glasses are capable of controlled and sustained release of antimicrobial ions (e.g. Ag, Cu, Zn, Ce and Ga) and thus have the potential to allow localized antimicrobial treatments which are advantageous compared to systemic antibiotic delivery systems. Osteogenesis and angiogenesis properties are also promoted with the release of some of the therapeutic ions, e.g. Cu, Zn, Ce and Ga [11], [37], [102], [116], [120]. In low concentrations silver has no toxicity effect to human cells, and it can also give antibacterial, osteoconductivity and bone bonding properties to BGs [81], [82], [83], [84], [85].Copper ion is widely used due to its vascularization/angiogenesis stimulation properties [133]. Zinc is a cofactor in metabolic processes in bone, articular tissues and immune system functions [110], [134], [135]. Bone formation and mineralization are stimulated by activating aminoacyl-tRNA synthetase in osteoblastic cells via the zinc doping of BGs [116]. Cerium has been shown to enhance the proliferation, differentiation and mineralization of primary osteoblasts [136]. Besides promoting osteogenesis, cerium incorporated materials have demonstrated promising antibacterial properties [120]. Gallium ions are effective against bone desorption and for the treatment of osteoporosis and cancer related hypercalcemias [137], [138]. Ga is also effective against organisms causing tuberculosis and malaria in human beings as well as against Pseudomonas aeruginosa [139], [140]. Moreover, besides limiting bacteria attachment, these ions can inhibit bacteria replication by damaging bacteria RNA and DNA, i.e. silver ions, or decreasing bacterial Fe uptake, i.e. gallium ions. Silver has been used more than the other antibacterial ions (Cu, Zn, Ce and Ga) as it has a broad and strong antimicrobial behavior and has no toxicity effect to human cells in suitable concentrations, thus providing extra functions to BGs in addition to osteoconductivity and bone bonding ability.