1. Introduction
Long before being isolated as a proper element by the Swedish chemist Georg Brandt (c. 1735), cobalt metal and cobalt minerals have been used during the Antiquity as blue pigments (cobalt blue) for jewellery, pottery and paintings, and to impart a distinctive blue tint to glass. The name kobold, meaning ‘mischievous spirit’, was first applied (16th century) by Schneeberg miners in the Hartz Mountain of Saxony (Germany) who were looking for precious metals from ores thought to contain copper. These ores were eventually determined as containing poisonous cobalt arsenides, whereby arsenic species evaporated during roasting (Peek et al., 2009). Cobalt can also be found in varying amounts in plants, soils and animals and is a vital element for many organisms. With mammals, cobalt is an active component of vitamin B12 (cobalamin), a substance which catalyses the regeneration of red blood cells, and plays a role in the normal functioning of the brain and nervous system (Hawkins, 2001).
Cobalt (symbol Co) has unique properties which give it many desirable features: (1) unique valency properties with variable oxidation state, explaining its wide use as a catalyst (Cobalt Development Institute, 2016a); (2) high metal hardness which can retain its strength and corrosion resistance over a wide temperature range up to its melting-point (1495 °C), which is critical for imparting strength when forming alloys with other metals at high temperature (Peek et al., 2009) and (3) ferromagnetic properties allowing it to be magnetised. In addition, it has the highest known Curie point (1121 °C), meaning that its magnetic properties are retained to this temperature and can hence be used to produce permanent magnets. As a consequence of these unique physical properties, including heat resistance, strength and magnetic properties, cobalt has a wide variety of applications.
It was only in the early 20th century that cobalt began to be used in various alloys and as a catalyst in the chemical industry, resulting in a rapid increase in production towards the mid-20th century. In the 1990′s, the dominant application of cobalt was in the production of very hard superalloys which had distinct magnetic properties and high temperature resistance (Seddon, 2001). End-uses of cobalt-bearing superalloys are found in the aerospace industry, land-based and marine turbines and various industrial, medical, automotive and defence-related applications. Nowadays, the global cobalt market is dominated by the rechargeable battery industry which is responsible for 58% of cobalt use (Darton Commodities Ltd., 2020). The battery industry only represented 10% of the cobalt market share in 1999 (Seddon, 2001), and growth of the battery market is due to the emergence of smartphones, tablets and laptops, and the large batteries required for Electric Vehicles (EV) and Plug-in Hybrid EV (PHEV). This trend is expected to continue into the foreseeable future regardless of battery chemistry: the demand for rechargeable batteries is expected to increase in line with governmental targets, policy supports and the need to strengthen the global response to the threat of climate change as reaffirmed by the Paris Agreement (COP21) in 2015 (Darton Commodities Ltd., 2020).
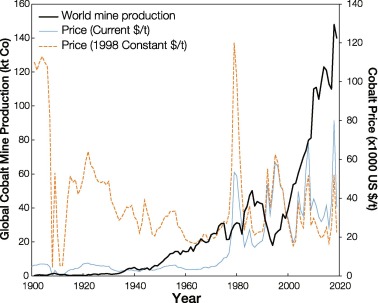
Over the last century, diversification of cobalt applications has led to an increase of cobalt production, with a few ups and downs due to political instability and fluctuations in the international market price (Fig. 1). In 2019, over 140 kt Co was produced from primary sources worldwide, with ~70% (100 kt Co) being produced by, or originating from, the Democratic Republic of Congo (DRC). Production of cobalt in DRC has been a dominant proportion of global supply for several decades (USGS, 2020). Schmidt et al. (2016) estimated that almost 100% of cobalt chemicals (oxides, hydroxides, carbonates and sulphates) are produced from the weathered stratiform sediment-hosted Cu-Co ore of the DRC. Cobalt metal (cathodes, briquettes, powder) is produced from nickel sulphide ores (49%), copper sulphides ores (32%) and nickel laterites (limonites only, 19%). The production of refined cobalt metal is currently dominated by China (~67%), followed by Finland (~11%) and Canada (~5%). This implies that more than two-thirds of refined cobalt is produced in countries where no cobalt mining takes place (Darton Commodities Ltd., 2020, Roberts and Gunn, 2014). Statistics suggest that less than 10% of global mined production is actually refined within the country of origin, implying that cobalt production is often based purely on imported materials. It is estimated that about 69% of cobalt used in manufactured products is sent to landfills. About 22% of the discarded cobalt is collected for scrap markets and the remainder is downgraded for other scrap markets (Harper et al., 2012). Given the growing importance of devices which require cobalt, combined with the relatively high supply risk and low recycling rate of cobalt, the European Union's Raw Materials Initiative classified cobalt as a critical element (European Commission, 2014).
 Fig. 1
Fig. 1Due to its low concentration in ores, cobalt is usually considered to be a companion metal which is of limited economic interest. The bulk of global cobalt production emanates as a by-product of extraction of commodities such as copper (~55%), nickel (~35%), and arsenic (Azevedo et al., 2018, Smith, 2001). The only exceptions, which represent a small share of the global cobalt production, are the active Bou Azzer mine in Morocco (Leblanc and Billard, 1982) and the now closed Cobalt mine in Ontario, Canada, the Black Bird district in Idaho, United States (Smith, 2001), and Mount Cobalt mine in Queensland, Australia (Croxford, 1974). A consequence of the principal focus resting on other commodity is a relatively low and variable recovery efficiency for cobalt (De Cuyper, 1981), which often reports to tailings or smelter slags (Shedd, 1993). Whether cobalt can be profitably recovered as a by-product depends on factors such as the amount of cobalt that can be mined and recovered as a marketable product, typical recovery efficiencies for this companion metal (especially for the different technologies used), and the relative cost and benefit of cobalt metal recovery (Mudd et al., 2013). All these factors are a complex function of key ore properties (e.g. hardness, leachability, acid consumption, impurity levels, floatability, etc.) which are directly linked to the type of deposit and ore mineralogy (Baum, 1999, Baum and Ausburn, 2013, Macfarlane and Williams, 2014, Peek et al., 2009).
Geometallurgy is a multi-disciplinary holistic approach that aims to improve resource management by integrating geological, mining, metallurgical, environmental and economic information to generate a database that can be readily integrated into a near real-time spatial predictive model for mineral processing plants of future or existing mining operations (Michaux and O’Connor, 2020, Williams and Richardson, 2004). This requires detailed characterisation of the geological, mineralogical, geochemical, and geotechnical ore characteristics which are critical for optimising resource efficiency, metallurgical processes performance and reducing technical risks (Dehaine et al., 2019b, Lund and Lamberg, 2014).
The purpose of this paper is to present a comprehensive review of the geometallurgy of cobalt-bearing deposits, including an analysis of cobalt geochemistry, geology, mineral resources and production. The starting point is a review of the mineralogical and geometallurgical properties of cobalt ores, followed by a description of the main processing routes. For the three main cobalt producing deposit types (sediment hosted Cu-Co, Ni-Co laterite, and magmatic Ni-Cu-Co sulphide deposits), the geometallurgical challenges are discussed with emphasis on the link between key ore properties and processing performance. Finally, the future of cobalt mineral extraction is addressed in terms of cobalt resources, cobalt extraction efficiency and processing technology, as well as socio-environmental aspects related to cobalt extraction.
2. Geochemistry of cobalt
Cobalt is a lustrous greyish, silver, brittle transition metal with atomic number 27. It is surrounded in the Periodic Table by iron and nickel which display similarity in physical properties. Cobalt has only one naturally-occurring stable isotope, Co59, and twelve known radioactive isotopes, of which Co60 is the most commonly exploited. The most common oxidation state of cobalt is 2+, while 3 + and 1 + may occur. The ionic radius of Co2+ is 0.72 Å and 0.63 Å for Co3+, both of which are similar to the ionic radii of Cu2+ (0.72 Å), Mg2+ (0.65 Å), Mn2+ (0.80), Fe2+ (0.74 Å), Fe3+ (0.64 Å), and Ni2+ (0.69 Å). This allows cobalt to substitute for these elements within many minerals and other phases under favourable conditions (Young, 1957). Cobalt is not particularly reactive, and it is stable in air at normal temperatures. Upon heating, it first oxidises to Co3O4 and then, above 900 °C, to CoO (Cobalt Development Institute, 2016a). Co2+ is stable in acidic solution in the absence of complexing agents, but it is more readily oxidised to Co3+ in alkaline solution or, in any solution, in the presence of complexing agents; Co3+ has a high affinity for complex formation (Donaldson et al., 2005). Under oxidizing conditions, cobalt shows a strong tendency to concentrate with manganese oxides. During the weathering of mafic and ultramafic rocks, manganese oxides and cobalt tend to accumulate in the upper levels of a deposit, while nickel moves downwards with magnesia and silica to form laterites (Donaldson et al., 2005, Young, 1957).
Cobalt is a fairly common element in Earth’s crust, averaging about 17.3 ppm (Rudnick and Gao, 2013), which ranks it as element no. 33 in terms of crustal abundance (Cobalt Development Institute, 2016a). Cobalt is found in relatively high concentrations in mafic and ultramafic igneous rocks (Table 1). The Ni/Co ratio decreases from ultramafic to acidic rocks because nickel enters the lattice of early crystallising magnesium silicates more readily than cobalt. On the other hand, the Cu/Co ratio globally increases from ultramafic to acidic rocks (Donaldson et al., 2005). In sedimentary rocks, cobalt is mostly distributed in the argillaceous fraction and seems to follow Fe and Mn (Carr and Turekian, 1961), with varying amounts of cobalt depending on rock type, i.e. average cobalt content of 19, 0.3 and 0.1 ppm for shales, sandstone and carbonates respectively (Krauskopf and Bird, 1995).
Table 1. World average cobalt concentrations in some igneous, sedimentary and metamorphic rocks.
| Rock type | Co content (ppm) | Ni/Co ratio | Cu/Co ratio | Reference |
|---|---|---|---|---|
| Igneous rocks | ||||
| Ultramafic | 200.0 | 10.0 | 0.1 | (Krauskopf and Bird, 1995) |
| Dunite | 108.6 | 21.5 | 0.2 | (Gülaçar and Delaloye, 1976) |
| Pyroxenite | 55.2 | 8.1 | 5.1 | (Gülaçar and Delaloye, 1976) |
| Serpentinite | 115.1 | 18.2 | 0.7 | (Gülaçar and Delaloye, 1976) |
| Mafic | 45.0 | 3.6 | 2.2 | (Krauskopf and Bird, 1995) |
| Gabbro | 51.0 | 2.6 | – | (Donaldson et al., 2005) |
| Basalt | 41.0 | 2.5 | – | (Donaldson et al., 2005) |
| Diabase | 47.0 | 1.6 | 2.3 | (Krauskopf and Bird, 1995) |
| Intermediate igneous | 10.0 | 5.5 | 3.5 | (Krauskopf and Bird, 1995) |
| Felsic | 5.0 | 1.6 | 4.0 | (Krauskopf and Bird, 1995) |
| Granite | 47.0 | 0.4 | 5.4 | (Krauskopf and Bird, 1995) |
| Sedimentary rocks | ||||
| Shales | 19.0 | 3.6 | 2.4 | (Krauskopf and Bird, 1995) |
| Sandstone | 0.3 | 6.7 | 1.0 | (Krauskopf and Bird, 1995) |
| Carbonates | 0.1 | 200.0 | 1.0 | (Krauskopf and Bird, 1995) |
| Metamorphic rocks | ||||
| Schists | 40.0 | – | – | (Carr and Turekian, 1961) |
| Quartzite | 0.3 | – | – | (Carr and Turekian, 1961) |
Pure cobalt does not exist in nature, but cobalt is present as an essential constituent in about 66 minerals, as recorded in the International Mineralogical Association’s (IMA) RRUFF database (Lafuente et al., 2015), and as a minor or trace constituent of several hundred more, particularly those containing nickel, iron and manganese (Donaldson et al., 2005). Cobalt can sometimes be found in economic concentrations in olivine, spinel and chlorite in lateritic and hydrothermal deposits (Smith, 2001). By comparison, nickel and copper are essential elements in over 160 and 710 minerals, respectively (Lafuente et al., 2015). This relative scarcity of cobalt-only minerals is explained by its chalcophile and siderophile properties, which is a consequence of cobalt’s charge and ionic radius being very close to that of more common mineral-forming elements such as iron, nickel and manganese, making it easier for cobalt to incorporate as a minor element in abundant rock-forming minerals than to be isolated into its own species (Hazen et al., 2017, Roberts and Gunn, 2014).
3. Cobalt geology and resources
3.1. Cobalt geology and mineral deposit type
Cobalt mineralisation is typically found in Cu-Co sulphide or oxide ores, Ni-Cu magmatic sulphide ores or Ni-Co laterites. Although the level of detail recorded in the literature varies, cobalt is known to be present in many deposit types (Crockett et al., 1987, Mudd et al., 2013, Petavratzi et al., 2019, Slack et al., 2017, Smith, 2001, Sverdrup et al., 2017). Slack et al. (2017) defined 8 major terrestrial deposit types. This review mostly discusses the 3 major deposit types from which cobalt is commercially extracted at large scale, viz. stratiform sediment-hosted Cu-Co deposits, Ni-Co laterites, and magmatic Ni-Cu-Co sulphide deposits. The remaining deposit types are regrouped in the hydrothermal and volcanogenic deposits category while marine-based resources will be discussed in the future trends section (section 6.1.1.) as these are relevant for future cobalt production.
3.1.1. Stratiform sediment-hosted (SSH) Cu-Co deposits
Cobalt occurrences of this type are mostly confined to adjacent regions of the DRC and Zambia in the Central African Copperbelt (CAC) which host the world’s greatest concentrations of copper and cobalt (Cailteux et al., 2005a). Cobalt is present in siliciclastic and carbonate sedimentary rocks and volcanic and plutonic mafic rocks of the Katangan supracrustal sedimentary succession, which are emplaced in a continental rift. In Zambia, copper-cobalt deposits are hosted in para-autochthonous siliciclastic rocks. In the DRC, the main lithological units are dolomitic limestones and dolomite-rich shales, and the deposits and host rocks form thrusts sheets within the Lufilian arc (Cailteux et al., 2005b, Kampunzu et al., 2000).
In general, there are three zones: a weathered oxide zone extending to a depth between 70 and 150 m, followed by a mixed oxide-sulphide zone, and a sulphide zone that extends at greater depths than 250 m (Crundwell et al., 2011a). The weathering process is mainly observed in the DRC, where the effect of meteoric fluids has produced cobalt enrichment in the upper part of the weathered zone (Roberts and Gunn, 2014). The most abundant oxidised cobalt mineral is heterogenite (Roberts and Gunn, 2014) while, at depth, cobalt is mainly present in the sulphide mineral carrollite (Dewaele et al., 2006). The thickness of the ore bodies varies from a few to several tens of meters, with varying cobalt content. Lateral metal zonation in the ore bodies means that high cobalt and copper values do not necessarily coincide (Guilbert and Park, 2007).
3.1.2. Ni-Co Laterite deposits
The second most important class of deposits consists of mid-Tertiary to recent laterites. Laterites are formed by deep tropical weathering of bedrock, during which certain elements are removed and others enriched by supergene processes (Smith, 2001). While these deposits mainly contain significant amounts of nickel, appreciable amounts of cobalt may be present (Berger et al., 2014). There are three types of nickel–cobalt laterite deposits: (a) hydrous silicate deposits with a top layer of oxide laterites, under which hydrous magnesium-nickel silicates occur in the lower saprolite, (b) clay silicate deposits, where smectitic clays have developed in the mid or upper saprolite, and (c) limonite deposits, where altered bedrock is overlain by iron oxyhydroxides. The type of laterite deposit which is formed depends on the climatic conditions (Gleeson et al., 2003). Cobalt is concentrated when primary sulphide and silicate ore minerals are subjected to chemical and physical changes associated with atmospheric leaching. Where laterites have developed over a substratum of mafic or ultramafic igneous rocks, nickel and sometimes cobalt, is frequently concentrated in the weathered zone (Crockett et al., 1987). If the rocks are ultramafic in character, the concentration of nickel and cobalt can be up to ten times the concentration of the original bedrock (Robb, 2005). In general, the thickest layers of laterite with the highest concentrations of nickel, iron and cobalt tend to occur where the bedrock is characterized by closely-spaced jointing. This geological feature promotes maximum groundwater circulation and fluid-rock interaction over time. High-grade laterites display a topographic control and tend to occur beneath a hill or on the edge of a plateau or terrace, due to fluctuations in the water table levels and surface erosion (Robb, 2005). For the development of a substantial nickel–cobalt laterite deposit stable geological conditions are required and the rate of chemical weathering needs to be higher than the rate of physical erosion (Roberts and Gunn, 2014).
3.1.3. Magmatic Ni-Cu-Co sulphides deposits
Magmatic deposits are concentrations of nickel, copper and minor platinum group metals (PGMs) and cobalt produced by high temperature magmatic processes (Naldrett, 1997). Of main importance for cobalt are mafic or ultramafic intrusions (Crockett et al., 1987) and ultramafic volcanic flows i.e., komatiites (Lesher and Keays, 2002). Most of the deposits occur as conformable layers and lenses that occupy depressions in the base of the magmatic host bodies. The deposits and their host rocks are a result of mantle-derived mafic magmas that have assimilated crustal sulphur (Naldrett, 1999). Addition of externally derived sulphur caused the formation of immiscible sulphide melt that, being denser than the silicate melt, settled to the bottom of the magma chamber or magma conduit (Lesher and Keays, 2002, Robb, 2005). Ores that have undergone subsequent tectonic deformation and remobilisation occur as elongated lenticular masses and veins of sulphide-matrix breccia (Smith, 2001). In general, nickel is the main economic commodity in magmatic deposits. Copper can be present as co-product or by product, with cobalt as a minor by-product and with platinum group elements occurring in some deposits as by-products or even as the main commodity (Crockett et al., 1987, Crundwell et al., 2011b, Smith, 2001). Typical major sulphide minerals that may be present in these deposits are pentlandite, chalcopyrite and pyrrhotite. The cobalt grade typically varies between 0.04% and 0.08% Co (Roberts and Gunn, 2014).
3.1.4. Hydrothermal and volcanogenic deposits
These deposit are grouped under the same heading, even though hydrothermal and volcanogenic deposits can show a wide range of formation styles, occurring metals, minerals and grades. Notable examples are Cu (-Zn-Co-Ag-Au) Volcanogenic Massive Sulphides (VMS), Iron Oxide Copper Gold (IOCG) -Ag-U (-REE-Co-Ni), Fe-Cu skarn, Mississippi Valley Type (MVT) Zn-Pb (–Co-Ni) sulphide and polymetallic (Ag-Ni-As-Bi) –Co-rich veins deposit (Slack et al., 2017). The latter are the only deposits from which cobalt is extracted as the main commodity. A notable and unique example is the Bou Azzer deposit in Morocco. The deposit consists of cobalt–nickel-iron arsenides, with additional sulpharsenides, copper sulphides, molybdenite and gold in a quartz and carbonate gangue. The ore has undergone several phases of brecciation and recrystallisation related to Pan-African and Hercynian deformations which resulted in a wide range of shapes: lodes, veins, stocks, complex shells and flat lenses (Smith, 2001). The cobalt mineralisation is developed by the leaching of serpentinites by magmatic fluids, under reducing conditions and with high fluid/rock ratios. The deposition of ore mineralisation took place in response to an increase in pH (Ahmed et al., 2009). Another example of hydrothermal cobalt mineralisation is the Idaho Cobalt belt and the Blackbird district. The complex geological history makes the classification of these deposit types somewhat arbitrary (Crockett et al., 1987) but a recent classification regroups these deposits as metasediment-rock hosted Cu-Co-Au (Slack et al., 2017).
3.2. Cobalt mineral resources and ore reserves
Table 2 summarises the total reserves and mineral resources (measured + indicated + inferred) per deposit type based on data in a report on cobalt published by the US Geological Survey (Slack et al. , 2017). This assessment of terrestrial resources, updated with the latest resources estimates from the relevant mining companies, is by no means complete since many deposits known to contain cobalt are not reported but it gives a good estimate of current cobalt mineral resources and ore reserves. It states that around 95% of the world cobalt resources are contained in a) stratabound sediment- or shale-hosted Cu-Co deposits (~58%), which are mostly located in the Democratic Republic of Congo (DRC) and Zambia, b) lateritic Ni-Co deposits (~29%) located in Australia, New Caledonia and Cuba, and c) magmatic Ni-Cu-PGE-Co deposits (~9%) found in Australia, Canada, Russia, Finland and the United States. If the substantial marine sea floor resources were included in this assessment, these would represent around 80% of the total cobalt resources, according to the current geological endowment-based resource estimates (Slack et al., 2017). According to Table 2, the global cobalt resource is about 154 Mt Co, of which 121 Mt Co derives from sea-floor resources. The total tonnage of recoverable cobalt, calculated using an assumed cobalt recovery per deposit type, varies between 15.9 Mt Co (Mudd et al., 2013) and 34 Mt Co (Sverdrup et al., 2017). This difference is explained by the assumptions used in the two studies: the higher value was obtained by assuming that technology will be developed to convert cobalt ocean deposits into resources (Sverdrup et al., 2017), while the lower value was based only on extraction of cobalt from terrestrial resources. A summary list of the main cobalt-hosting mineral deposits, including historic and active or some advanced exploration projects is given in Table 3. The location of these deposits across the globe is shown in Fig. 2.
Table 2. Summary statistics of total cobalt reserves and mineral resources (measured + indicated + inferred) of cobalt-containing mineral deposit types based on data from Slack et al. (2017) updated with data from companies annual and quarterly reports when available.
| Deposit type/sub-type | Examples | Count | Total tonnage (Mt) | Avg. Co grade (%) | Total Co (Mt) | Share terrestrial (%) | Share all (%) |
|---|---|---|---|---|---|---|---|
| Strat. Sediment/ Shale-Hosted (SSH) Cu-Co | Mutanda (DRC), Sotkamo (Finland) | 61 | 11 060 | 0.29 | 19.16 | 58.2 | 12.4 |
| Ni-Co Laterite | Nkamouna (Cameroon), Jakaré (Brasil) | 82 | 11 202 | 0.08 | 9.31 | 28.5 | 6.1 |
| Magmatic Ni-Cu sulphide | Sudbury basin (Canada), Norilsk (Russia) | 42 | 12 194 | 0.07 | 2.81 | 8.5 | 1.8 |
| Hydrothermal and volcanogenic | 46 | 2 885 | 0.19 | 1.57 | 4.8 | 1.0 | |
| Cu (-Zn-Co-Ag-Au) VMS | Outokumpu (Finland), Windy Craggy (Canada) | 20 | 919 | 0.13 | 0.69 | 2.1 | 0.4 |
| IOCG-Cu-Au(-Ag-U-REE-Co-Ni) | Olympic Dam (Australia) | 10 | 1 067 | 0.04 | 0.29 | 0.9 | 0.2 |
| Metased.-rock-hosted Co-Cu-Au | Blackbird district (USA), NICO (Canada) | 8 | 115 | 0.23 | 0.26 | 0.8 | 0.2 |
| Fe-Cu-Co skarn and replacement | Cornwall (USA), Magnitogorsk (Russia) | 3 | 760 | 0.02 | 0.15 | 0.5 | 0.1 |
| Polymetallic (Ag-Ni-As-Bi)–Co-rich veins | Bou-Azzer (Morocco), Cobalt-Gowganda (Canada) | 3 | 23 | 0.92 | 0.14 | 0.4 | 0.1 |
| MVT Zn-Pb (–Co-Ni) sulphide | Mine La Motte-Fredericktown, Madison (USA) | 2 | 2 | 0.27 | 0.04 | 0.1 | <0.1 |
| Sea-floor Fe-Mn(-Ni-Cu-Co-Mo) nodules | CCZ, Pacific prime crust zone | 8 | 35 936 | 0.38 | 121.41 | – | 78.7 |
| Total | 239 | 73 277 | – | 154.34 | 100.0 | 100.0 |
Table 3. Total reserves and mineral resources (measured + indicated + inferred) of a selection of major cobalt-hosting mineral deposits, active mines or exploration projects (Darton Commodities Ltd., 2020, Mudd et al., 2013, Petavratzi et al., 2019, Slack et al., 2017). Data based on the most recent company mineral resources estimates (quarterly/annual reports) available. The location of the deposits with corresponding number can be found on the world map in Fig. 2.
| Operation Name | Deposit type | Country | Main Commodity | Tonnage (Mt) | Co grade (%) | Co (kt) | Current Owner | Stage | Map # |
|---|---|---|---|---|---|---|---|---|---|
| BOSS Mininga, b | SSH | DRC | Cu | 75.1 | 0.20 | 150 | Eurasian Resources Group | On care | 1 |
| Comidea | SSH | DRC | Cu | 53.0 | 0.20 | 106 | Eurasian Resources Group | Active | 2 |
| Etoilea, c | SSH | DRC | Cu | 21.7 | 0.40 | 88 | Chemaf ltd, Shalina Resources | Active | 3 |
| KCCd | SSH | DRC | Cu | 574.0 | 0.51 | 2908 | Katanga Mining ltd, Glencore plc | Active | 4 |
| Musonoia | SSH | DRC | Cu | 32.1 | 0.90 | 289 | Jinchuan Group | Project | 5 |
| Mutanda | SSH | DRC | Cu | 918.0 | 0.39 | 3584 | Glencore plc | On care | 6 |
| Mutoshi | SSH | DRC | Cu | – | – | 300 | Chemaf ltd, Shalina Resources | Project | 7 |
| Ruashi | SSH | DRC | Cu | 33.4 | 0.30 | 100 | Jinchuan Group | Active | 8 |
| Tenke Fungurume | SSH | DRC | Cu | 1015.6 | 0.29 | 2919 | China Molybdenum Company ltd | Active | 9 |
| Sotkamoa | SSH | Finland | Ni, Zn, Cu, | 1525.0 | 0.02 | 290 | Terrafame | Active | 10 |
| Boleo | SSH | Mexico | Cu | 424.6 | 0.05 | 221 | Korea Resources Corp. | Active | 11 |
| Bornitea | SSH | United States | Cu | 182.4 | 0.02 | 35 | Trilogy metals inc. | Project | 12 |
| Iron Creeka | SSH | United States | Cu | 4.8 | 0.24 | 12 | First Cobalt | Project | 13 |
| Konkola (KCM) | SSH | Zambia | Cu | 12.4 | 0.34 | 42 | Vedanta Resources plc, ZCCM plc | Active | 14 |
| Mopania | SSH | Zambia | Cu | 361.0 | 0.08 | 289 | Glencore plc | Active | 15 |
| Nova-Bollinger | Magmatic | Australia | Ni, Cu | 24.9 | 0.07 | 16 | Independence Group (IGO) | Active | 16 |
| Fortaleza de Minase | Magmatic | Brazil | Ni | 10.3 | 0.20 | 21 | Votorantim | Suspended | 17 |
| Santa Ritaf | Magmatic | Brazil | Ni, Cu | 159.3 | 0.02 | 24 | Mirabela Nickel | Closed | 18 |
| Raglan | Magmatic | Canada | Ni, Cu, PGE | 46.0 | 0.07 | 31 | Glencore plc | Active | 19 |
| Sudbury (Glencore) | Magmatic | Canada | Ni | 69.0 | 0.04 | 28 | Glencore plc | Active | 20 |
| Sudbury (Vale)f | Magmatic | Canada | Ni, Cu, PGE | 61.7 | 0.03 | 19 | Vale | Active | 20 |
| Thompsone | Magmatic | Canada | Ni, Cu, PGE | 39.2 | 0.09 | 35 | Vale | Active | 21 |
| Voisey’s Bay | Magmatic | Canada | Ni, Cu | 55.4 | 0.09 | 49 | Vale | Active | 22 |
| Jinchuane | Magmatic | China | Ni, Cu, PGE | 515.0 | 0.02 | 98 | Jinchuan Group | Project | 23 |
| Kevitsa | Magmatic | Finland | Ni, Cu, PGE | 286.3 | 0.01 | 30 | Boliden | Active | 24 |
| Sakattia | Magmatic | Finland | Cu, Ni, PGE | 44.4 | 0.05 | 20 | Anglo American | Project | 25 |
| Noril’sk areae | Magmatic | Russia | Ni, | 1309.0 | 0.06 | 785 | Norilsk Nickel | Active | 26 |
| Nkomati | Magmatic | South Africa | Ni, Cu, PGE | 302.1 | 0.02 | 60 | African Rainbow Minerals | Active | 27 |
| Kabangaa | Magmatic | Tanzania | Ni | 58.2 | 0.20 | 116 | Glencore plc | Active | 28 |
| Eagle | Magmatic | United States | Ni, Cu, Au, Ag | 4.3 | 0.067 | 4 | Lundin Mining | Active | 29 |
| Murrin-Murrin | Laterite | Australia | Ni | 332.7 | 0.08 | 261 | Minara Resources, Glencore plc | Active | 30 |
| Musgravea | Laterite | Australia | Ni | 215.8 | 0.07 | 151 | Metals X ltd | Project | 31 |
| Owendalea | Laterite | Australia | Ni, Sc | 16.9 | 0.12 | 20 | Platina Resources ltd | Project | 32 |
| Ravensthorpeg | Laterite | Australia | Ni | 535.8 | 0.03 | 149 | First Quantum Minerals ltd | On Care | 33 |
| Sunrise | Laterite | Australia | Ni, Sc | 101.0 | 0.12 | 129 | Clean Teq | Project | 34 |
| Barro Alto | Laterite | Brazil | Ni | 86.8 | – | – | Anglo American | Active | – |
| Jacaréa | Laterite | Brazil | Ni | 185.1 | 0.19 | 352 | Anglo American | Project | 35 |
| Niquelandiae | Laterite | Brazil | Ni | 56.3 | 0.06 | 34 | Anglo American | Active | 36 |
| Vermelhoa | Laterite | Brazil | Ni | 148.8 | 0.05 | 74 | Horizonte Minerals plc | Project | 37 |
| Musongatia | Laterite | Burundi | Ni | 150.0 | 0.09 | 135 | Kermas Group, State of Burundi | Active | 38 |
| Nkamouna | Laterite | Cameroon | Ni | 391.3 | 0.22 | 860 | Geovic Mining corp. | Project | 39 |
| Cerro Matoso | Laterite | Colombia | Ni | 95.0 | 0.10 | 95 | South 32 | Active | 40 |
| Moa Bay | Laterite | Cuba | Ni | 89.7 | 0.12 | 110 | Sherritt, General Nickel | Active | 41 |
| Cyclops Ni-Coh | Laterite | Indonesia | Ni | 37.0 | 0.11 | 41 | Pacific Rim Cobalt corp. | Project | 42 |
| Sorowako | Laterite | Indonesia | Ni | 116.5 | – | – | Vale | Active | – |
| Ambatovy-Analamay | Laterite | Madagascar | Ni | 251.3 | 0.08 | 201 | Sherritt | Active | 43 |
| Goroe | Laterite | New Caledonia | Ni | 323.0 | 0.11 | 355 | Vale NC | Active | 44 |
| Ramu | Laterite | Papua New Guinea | Ni | 175.0 | 0.10 | 175 | Highlands Pacific | Active | 45 |
| Agataa | Laterite | Philippines | Ni | 45.2 | 0.05 | 23 | Mindoro Resources ltd | Project | 46 |
| Beronge | Laterite | Philippines | Ni | 18.4 | 0.08 | 15 | Toledo Mining, DMCI holdings | Active | 47 |
| Mindoroe | Laterite | Philippines | Ni | 315.0 | 0.06 | 189 | Intex Resources | Project | 48 |
| Rio Tuba (Coral Bay)e | Laterite | Philippines | Ni | 57.7 | 0.12 | 69 | Sumitomo | Active | 49 |
| Taganito/Adlaye | Laterite | Philippines | Ni | 123.0 | 0.12 | 143 | Sumitomo | Active | 50 |
| Çaldağe | Laterite | Turkey | Ni | 37.9 | 0.05 | 19 | VTG Nickel | Project | 51 |
| Olympic Dame | Hydro. & volc. | Australia | Cu, U, Au, Ag | 605.0 | 0.02 | 121 | BHP Biliton | Active | 52 |
| NICO | Hydro. & volc. | Canada | Au | 64.0 | 0.12 | 74 | Fortune Minerals | Project | 53 |
| Kylylahti | Hydro. & volc. | Finland | Cu, Ni | 8.1 | 0.12 | 10 | Boliden | Closing | 54 |
| Rompas-Rajapalota | Hydro. & volc. | Finland | Au | 4.3 | 0.04 | 2 | Mawson Gold ltd | Project | 55 |
| Bou Azzer (district)e | Hydro. & volc. | Morocco | Co, As | 45.7 | 1.50 | 686 | CTT, Managem s.a. | Active | 56 |
| Blackbird (district)e | Hydro. & volc. | United States | Cu | 16.8 | 0.74 | 124 | Noranda Mining co. | Closed | 57 |
| Idaho Cobalt Operation | Hydro. & volc. | United States | Co | 5.7 | 0.55 | 31 | Jervois Mining ltd | Project | 58 |
- a
-
Mineral resources only.
- b
-
Include Kakanda, Mukondo, Kavundi central, Taratara, Saafi, Kababankola, Disele Sud, Luita Est (Ecaille Sud), Bangwe Est.
- c
-
Include Etoile, Etoile extension and ore stockpiles.
- d
-
Kamoto group, includes Kamoto, T17, Mashamba, KOV, Kananga, Tilwezembe.
- e
-
Data from Slack et al. (2017).
- f
-
Mineral reserves only.
- g
-
Include stockpiled resources.
- h
-
Total historic estimate.
 Fig. 2
Fig. 24. Geometallurgical properties of cobalt ores and minerals
Mineralogy is the principal, and possibly most important, geometallurgical ore property which drives the strategy adopted during ore processing (e.g. leaching versus flotation, leaching agent, flotation collector, etc.). Although styles of mineralisation vary from one type of deposit to another, only a relatively small number of minerals have been mined or concentrated for their cobalt content. The beneficiation of cobalt depends mainly on the state of oxidation (i.e. oxidised/weathered or reduced/sulphides) and the relative proportion of cobalt to copper or nickel. However, mineralogical composition is not the only characteristic of interest. Indeed, other mineralogical properties that influence process performance are: (i) ore-gangue mineralogy (which influences acid consumption and flotation performance), (ii) mineral associations and liberation of cobalt-bearing minerals (which controls availability/susceptibility to leaching and flotation), and (iii) impurity content in the host and associated minerals (which influences cobalt winning and reduces final product quality).
4.1. Mineralogical ore properties
4.1.1. Cobalt minerals
Table 4 lists some commonly exploited cobalt-bearing minerals, along with their mineral group, chemical formulas, content of cobalt and companion metals (copper, nickel), physical properties and acid solubility data (where this information is known).
Table 4. Common cobalt-bearing minerals with average metals content, along with the most relevant physical and chemical properties (hardness, density, magnetic susceptibility and acid solubility data when available). Most important cobalt-bearing minerals recovered economically are highlighted. Compiled using data from Anthony et al., 2001, Hazen et al., 2017, Lafuente et al., 2015.
| Mineral name | Group | Formula | Avg. grade (wt%)1 | Hardness | Avg. density (g.cm−3) | Avg. magnetic susceptibility2 (10−6) | Acid solubility3 | Example deposits | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Co | Ni | Cu | HCl | HNO3 | H2SO4 | |||||||
| Primary minerals | ||||||||||||
| Skutterudite | Arsenide | (Co,Ni)As3-x | 17.95 | 5.96 | – | 5.5–6.0 | 6.50 | 151.8 | n/a | H | n/a | Skutterud Mines (Norway), Bou Azzer (Morocco) |
| Smaltite | Arsenide | (Co,Fe,Ni)As3-x, 0.5<x<1 | 28.20 | – | – | 5.5–6.0 | 6.50 | 38.0 | I | H | I | Bou Azzer (Morocco), Langis mine (ON, Canada) |
| Safflorite | Arsenide | (Co,Fe)As2 | 21.25 | – | – | 4–5 | 7.10 | 73.8 | n/a | n/a | n/a | Elizabeth mine (Romania) |
| Cobaltite | Sulpharsenide | CoAsS | 35.52 | – | – | 5.5 | 6.33 | 66.6 | I | H | I | Sudbury (Canada), Broken Hill, (NSW, Australia) |
| Alloclasite | Sulpharsenide | (Co,Fe)AsS | 26.76 | – | – | 5.0 | 6.17 | n/a | n/a | S | n/a | Elizabeth mine (Romania), Silverfields mine (ON, Canada) |
| Glaucodot | Sulpharsenide | (Co,Fe)AsS | 26.76 | – | – | 5.0 | 5.95 | 354.4 | n/a | D | n/a | Håkansboda (Sweden) |
| Carrollite | Sulphide | Cu(Co,Ni)2S4 | 28.56 | 9.48 | 20.53 | 4.5–5.5 | 4.65 | 108.6 | I | S | n/a | Chambishi, Mutanda (DRC), Carroll County (MD, USA) |
| Linnaeite | Sulphide | Co2+Co23+S | 57.95 | – | – | 4.5–5.5 | 4.80 | 532.0 | n/a | S | D | Bou Azzer (Morocco), Noril’sk (Russia) |
| Siegenite | Sulphide | (Ni,Co)3S4 | 14.51 | 43.36 | – | 5–5.5 | 4.65 | 162.7 | I | H | I | Jungfer Mine (Germany) |
| Cattierite | Sulphide | CoS2 | 47.89 | – | – | 4.5 | 4.80 | 1012.4 | n/a | n/a | n/a | Shinkolobwe (DRC) |
| Co-Pyrite | Sulphide | (Fe,Co,Ni)S2 | 13.90 | 0.19 | – | 6–6.5 | 5.02 | n/a | I | n/a | n/a | Outokumpu district (Finland) |
| Bravoite | Sulphide | (Fe,Ni,Co)S2 | 4.88 | 9.71 | – | 6.5 | 5.01 | n/a | I | S | S | Langis mine (ON, Canada), Bou Azzer (Morocco) |
| Willyamite | Sulphide | (Co,Ni)SbS | 20.78 | 6.90 | – | 5.5 | 6.76 | n/a | n/a | n/a | n/a | Broken Hill, (NSW, Australia) |
| Co-pentlandite | Sulphide | (Co,Ni,Fe)9 S8 | 54.18 | 15.69 | – | 4.5 | 5.22 | <1000 | n/a | n/a | n/a | Langis mine (ON, Canada), Sotkamo (Finland) |
| Secondary minerals | ||||||||||||
| Erythrite | Arsenate | Co3(AsO4)2·8(H2O) | 29.53 | – | – | 1.5–2.0 | 3.12 | 1660.2 | S | I | I | Bou Azzer (Morocco), Daniel Mine (Germany) |
| Roselite | Arsenate | Ca2(Co,Mg)(AsO4)2·2(H2O) | 9.95 | – | – | 3.5 | 3.69 | n/a | S | S | S | Rappold mines (Germany), Rosas mine (Sardinia, Italy) |
| Wendwilsonite | Arsenate | Ca2(Mg,Co)(AsO4)2·2(H2O) | 3.45 | – | – | 3.0–4.0 | 3.52 | n/a | n/a | n/a | n/a | Sterling Hill mine (NJ, USA) |
| Smolyaninovite | Arsenate | (Co,Ni,Mg,Ca)3(Fe3+,Al)2(AsO4)4·11(H2O) | 11.70 | 0.33 | – | 2.0 | 2.20 | 1060.2 | n/a | n/a | n/a | Mt Cobalt (Australia), Bou Azzer (Morocco) |
| Heterogenite | Oxide | CoO(OH) | 64.10 | – | – | 3–5 | 4.30 | 255.6 | S | n/a | S | Katanga province (DRC) |
| Asbolane | Oxide | (Ni,Co)2-xMn4+(O,OH)4·n(H2O) | 3.30 | 9.85 | – | 6.0 | 3.26 | n/a | S | n/a | S | Koniambo Massif, Goro (New Caledonia) |
| Co-Lithiophorite | Oxide | (Al,Li,Ni,Co)(Mn,Fe,Mg)O2(OH)2 | 1.90 | 1.90 | – | 2.5–3.0 | 3.30 | n/a | n/a | n/a | S | Tiébaghi (New Caledonia), Shinkolobwe (DRC) |
| Kolwezite | Carbonate | (Cu,Co)2(CO3)(OH)2 | 17.84 | – | 39.05 | 4.0 | 3.97 | n/a | n/a | n/a | S | Musonoi, Kamoto, Mupine and Mashamba West mines (DRC) |
| Sphaerocobaltite | Carbonate | CoCO3 | 49.55 | – | – | 3.0–4.0 | 4.10 | n/a | S | n/a | n/a | Tenke Fungurume (DRC), Schneeberg district (Germany) |
| Moorhouseite | Sulphate | CoSO4·6(H2O) | 13.46 | 6.70 | – | 2.5 | 1.97 | n/a | n/a | n/a | n/a | Magnet Cove mine (NS, Canada) |
| Bieberite | Sulphate | CoSO4·7(H2O) | 20.96 | – | – | 2.0 | 1.90 | n/a | S | n/a | n/a | Schneeberg district (Germany), Jáchymov District (Czech Republic) |
| Aplowite | Sulphate | (Co,Mn,Ni)SO4·4(H2O) | 15.66 | 2.60 | – | 3.0 | 2.33 | n/a | n/a | n/a | n/a | Magnet Cove mine (NS, Canada) |
| Freboldite | Selenide | CoSe | 42.74 | – | – | 2.5–3.0 | 7.70 | n/a | n/a | n/a | n/a | Steinbruch Trogtal quarry (Germany) |
| Trogtalite | Selenide | CoSe2 | 27.18 | – | – | 7.0 | 7.09 | n/a | n/a | n/a | n/a | Musonoi mine (DRC), Steinbruch Trogtal quarry, (Germany) |
| Penroseite | Selenide | (Ni,Co,Cu)Se2 | 8.14 | 16.22 | 2.93 | 2.5–3.0 | 6.66 | n/a | n/a | S | n/a | Pacajake mine (Bolivia) |
| Tyrrellite | Selenide | (Cu,Co,Ni)3Se4 | 10.59 | 3.52 | 22.84 | 3.5 | 6.60 | n/a | n/a | n/a | n/a | Goldfields District (SK, Canada), Petrovice deposit (Czech Republic) |
- 1
-
Average content from the Handbook of Mineralogy (Anthony et al., 2001), chemical composition may vary.
- 2
-
Converted in volume magnetic susceptibility in SI units after the data from (Agricola et al., 1985, Clifford and Higley, 1978, Dahlin and Rule, 1993, Krs and Kropáček, 1987, Powell, 1967, Smith et al., 1976). See Appendix A for details about the calculations.
- 3
-
Solubility in concentrated acid, S: Soluble, I: Insoluble, H: Soluble if heated, D: Decomposed, n/a: Non-tested (Clifford and Higley, 1978, Lange, 1944, Smith, 1953).
The most common primary cobalt ore minerals currently exploited are cobalt sulphides (carrollite, cattierite, linnaeite) representing the main source of cobalt in the DRC, sulpharsenides (cobaltite) found in Zambia, Canada and the United States, arsenides (skutterudite, smaltite) found in copper-cobalt ores in Ontario, Canada and Morocco, and arsenates (erythrite) mainly occurring in the Bou Azzer deposit in Morocco. Less frequently exploited primary cobalt minerals include cobalt selenides such as trogtalite which has been found in the Musonoï mine (Kolwezi, DRC) in association with various palladium selenides (Gauthier and Deliens, 1999, Pirard and Hatert, 2008).
Secondary cobalt minerals mostly result from alteration of primary cobalt-bearing phases by oxidative weathering, hydration or other forms of alteration (Hazen et al., 2017). The most common cobalt oxide is heterogenite, a hydrated metal oxide with a variable copper and cobalt composition. Heterogenite, which contains cobalt in both Co2+ and Co3+ oxidation states, accounts for the majority of the cobalt in the weathered SSH Cu-Co deposits (Decrée et al., 2015, Vanbrabant et al., 2013). Another common cobalt oxide mineral is asbolane, a hydrated manganese-cobalt nickel mineral which is the source of most cobalt in NewCaledonian laterite deposits (Llorca and Monchoux, 1991) but also in the supergene zones of sulphide deposits of the CAC (Vanbrabant et al., 2013) and ferromanganese crusts of the Magellan Seamount cluster (Glasby et al., 2007). Lithiophorite is a manganese oxide consisting of sheets of linked MnO6 octahedra alternating with sheets of (Li,Al)(OH)6 octahedra with a crystallographic structure very close to that of asbolane with which it often closely associated. Llorca and Monchoux (1991) concluded that asbolane and lithiophorite may be end-members of a continuous series. Despite its name, which suggests that lithium is an essential constituent of lithiophorite, its composition can vary, and lithium-free varieties may contain significant concentrations of cobalt (Chukhrov et al., 1985). Cobalt-rich lithiophorite is considered as an important host of cobalt in West Australian laterites (Tindall and Muir, 1996), while cobalt-rich lithiophorite has been reported in laterites from New Caledonia (Manceau et al., 1987), as well as in the weathered SSH Cu-Co deposits of the CAC (Vanbrabant et al., 2013). Cobalt carbonates (sphaerocobaltite, kolwezite) can occur in a primary phase crystalized from carbonate-rich solutions and in an alteration phase derived from primary cobalt minerals (Hazen et al., 2017). Cobalt carbonates are mostly found in the supergene zones of Cu-Co sulphide ore deposits, especially in CAC (Gauthier and Deliens, 1999, Pirard and Hatert, 2008) where these have misclassified as cobalt-rich dolomite (Barton et al., 2014). The hydrous carbonate kolwezite is a typical secondary cobalt mineral in SSH Cu-Co deposits in DRC, especially the Musonoï Principal, Kamoto, Mupine and Mashamba West mines.
Besides cobalt production from the aforementioned cobalt minerals, cobalt can be extracted from other minerals into which cobalt has been substituted. These include arsenopyrite, pyrrhotite, pyrite, pentlandite in magmatic Ni sulphides, oxides (limonite, goethite), clays (nontronite) in Ni Laterites, and carbonates (malachite, dolomite) in SSH Cu-Co deposits (Donaldson et al., 2005, Slack et al., 2017).
4.1.2. Associated minerals
The overall ore mineralogy and, in particular, the gangue composition plays a critical role during the processing of cobalt-containing ores. Gangue composition is sometimes a controlling factor for the selection of the processing route (i.e. flotation vs leaching) for oxide ores (Bulatovic, 2010). It is considered as the single most important parameter affecting operating costs and recoveries of hydrometallurgical projects (Jansen and Taylor, 2003). For instance, ores containing large amounts of acid consuming gangue minerals (i.e., dolomite, micas, and clays), cannot be leached economically due to excessive acid costs. There is also an appreciable difference in floatability between oxide minerals from carbonate and silicate ores, which require the use of specific flotation reagents (Bulatovic, 2010, Shungu et al., 1988). Elemental deportment between the different sulphide minerals and gangue mineral content is known to influence copper and cobalt recovery from SSH sulphide ores (Tijsseling et al., 2020). Highly weathered ores usually display a complex gangue mineralogy which can play a significant role in the overall flotation efficiency (Bafubiandi and Bell, 2000) and often result in large amounts of slimes which have a negative effect on the floatability of oxide copper minerals (Bulatovic, 2010). Even downstream processes like leaching-solvent extraction-electrowinning (L-SX-EW) can be disturbed when silica-rich ores are leached (Shungu et al., 1988). The following section describes some of the common gangue minerals associated with cobalt ores and issues these cause during processing. Table 5 summarises the main gangue minerals associated with cobalt ores, their most relevant properties, and their influence on the various processing stages.
Table 5. Major gangue minerals associated with cobalt ores and their most relevant properties with regard to mineral processing (Anthony et al., 2001, Jansen and Taylor, 2003, Macfarlane and Williams, 2014, Shengo et al., 2019, Tijsseling et al., 2020, Weise, 1985).
| Mineral | Formula | Hardness | Avg. density (g.cm−3) | Avg. BWI (kWh/t) | TAC (moles/mole mineral) a | IAC (kg/ton ore)b | Negatively impacted process | ||
|---|---|---|---|---|---|---|---|---|---|
| Comminution | Flotation | L-SX-EW | |||||||
| Quartz | SiO2 | 7 | 2.62 | 14.7 | I | ●● | |||
| K-Feldspar | KAlSi3O8 | 6 | 2.56 | 11.9 | I | 6.8 | ● | ||
| Ca-Feldspar | (Na,Ca)Al2Si2O8 | 6 | 2.73 | 11.9 | 4 | 7.5 | ● | ● | |
| Biotite | K(Mg,Fe)3(OH,F)2(Si3AlO10) | 2.5–3 | 3.09 | 3–5 | ● | ●● | |||
| Calcite | CaCO3 | 3 | 2.71 | 1 | 9.8 | ● | ● | ●●● | |
| Dolomite | CaMg(CO3)2 | 3.5–4 | 2.84 | 12.6 | ● | ●● | ●●● | ||
| Magnesite | MgCO3 | 4 | 3.00 | 12.5 | ●● | ●● | |||
| Pyrrhotite | Fe(1-x)S, x = 0–0.17 | 3.5–4 | 4.61 | 10.4 | ●● | ||||
| Pyrite | FeS2 | 6.5 | 5.01 | 9.7 | ● | ||||
| Limonite | FeO(OH) n H2O | 4–5.5 | ~3.6 | 9.0 | |||||
| Goethite | FeO(OH) | 5–5.5 | 3.80 | 3 + A | ● | ||||
| Hematite | Fe2O3 | 6.5 | 5.30 | 13.3 | ● | ||||
| Muscovite | KAl2(AlSi3O10)(OH,F)2 | 2–2.5 | 2.82 | I | ●● | ||||
| Smectite | Group | 1–2 | ~2.50 | ~7 | HA | 11.1 | ● | ● | |
| Illite | (K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2,(H2O)] | 1–2 | 2.75 | ~7 | 10.7 | ● | ● | ||
| Kaolinite | Al2Si2O5(OH)4 | 2–2.5 | 2.60 | HA | ●● | ● | |||
| Chlorite | (Fe,Mg,Al)6(Si,Al)4O10(OH)8 | 2–2.5 | 2.65 | 6 | 12.0 | ●● | ●● | ||
| Talc | Mg3Si4O10(OH)2 | 1 | 2.75 | HA | ● | ●●● | ●● | ||
| Serpentine | Group | 2.5–4 | ~2.50 | ●● | ● | ||||
- a
-
Theoretical acid consumption, may vary with mineral composition. A: adsorption, HA = High adsorption, I: Insoluble.
- b
-
Ideal acid consumption, after Youlton and Kinnaird (2013), may vary with mineral composition.
4.1.2.1. Quartz
Quartz is an abundant gangue mineral in most cobalt-bearing mineral deposits. It has a relatively high hardness of 7 on the Mohs scale and is known to be abrasive. Coarse-grained and abundant quartz increases the wear rate of mill linings and may increase operational costs (Jensen et al., 2010, Macfarlane and Williams, 2014) as well as negatively impact throughput due to higher Bond Work Index (BWi), see Table 5. Quartz is also largely insoluble in acid and considered to be an unreactive mineral phase during conventional acid leaching processes (Chetty and Deshenthree, 2018). When cobalt minerals are locked in quartz, a negative impact on leaching performance will be observed. Similarly, the presence of non-liberated or locked cobalt minerals in quartz will affect the cobalt recovery during flotation, with cobalt being lost to tailings. For both processing options, the only solution is to increase the specific surface area of ore particles by grinding the ore to a finer size.
4.1.2.2. Carbonates
Calcium and magnesium carbonate minerals (calcite, dolomite, magnesite and ankerite) are the second most common gangue minerals present in most cobalt-hosting mineral deposits, especially in sediment-hosted Cu-Co deposits. Dolomitic ores are generally harder and may require a change of mining technique from scraping to drilling and blasting (Macfarlane and Williams, 2014). Contrary to siliceous gangue minerals, the presence of carbonate gangue minerals affects the floatability of oxidic copper-cobalt minerals, making the use of the sulphidisation method necessary (Bafubiandi and Bell, 2000, Bulatovic, 2010, Shungu et al., 1988, Tijsseling et al., 2019).
Carbonates are well-known for consuming acid during leaching of oxidic cobalt minerals, with specific kinetics depending on the mineral species. Calcite dissolves completely in very diluted sulphuric acid at room temperature, whereas dolomite and ankerite are sparingly soluble, and only dissolve noticeably in heated solutions of acid (Chetty and Deshenthree, 2018). As shown by the reaction equation, dissolution of calcium carbonate in sulphuric acid is accompanied by the formation of gypsum and generation of carbon dioxide (Bingöl and Canbazoǧlu, 2004):CaCO3 (s) + H2SO4 (aq) + H2O ⇋ CaSO4·2H2O(s) + CO2(g)
Research suggest that 0.4 to 0.7 tonnes of H2SO4 per tonne of recovered copper may be required for oxidised copper ore containing malachite (Ambo, 2015, Bingöl et al., 2005). This would represent up to 25% of the operating cost associated with copper leaching (Baum and Ausburn, 2013). In line with uranium ore processing, it is estimated that the carbonate content should be below 1.5–2% to make leaching using sulphuric acid more economic that alkaline leaching. Above 15–20% CaCO3 in the ore, the cost linked to acid consumption would be prohibitive (Bowell et al., 2011). Besides acid consumption, emission of carbon dioxide into the atmosphere is a concern. It is estimated that a tonne of sulphuric acid produces approximately 450 kg of carbon dioxide when it reacts with carbonate gangue material (Mathew, 2013).
Processing options to improve economic and environmental performance linked to carbonate gangue minerals include: (i) removing most of the carbonate mineral content of the ore prior to leaching either by applying flotation sulphidisation with Mercaptan and/or dithiophosphate as secondary collectors for cobalt minerals (Bulatovic, 2010, Tijsseling et al., 2019), (ii) using depressants or applying reverse flotation of the carbonate gangue minerals prior to cobalt oxide minerals flotation (Dehaine et al., 2019a, Shengo et al., 2019) or (iii) preconcentration using sensor-bases sorting (Phiri et al., 2018).
4.1.2.3. Phyllosilicates and clay minerals
Phyllosilicates are a group of minerals which includes serpentinite, talc, mica, chlorite, and clay minerals such as kaolinite, illite, smectite and vermiculite. Often erroneously considered to be clay ‘only’, phyllosilicates are common gangue minerals in cobalt-containing ores. With nickel laterites, Cu-Co sediment-hosted and cobalt arsenide deposits, phyllosilicates are known to cause issues throughout the mineral processing circuit (Ndlovu et al., 2013). With flotation, the presence of phyllosilicate minerals increases reagent consumption and reduces selectivity through entrainment of slimes into the concentrate (Jorjani et al., 2011), increased pulp viscosity (Arnold and Aplan, 1986, Genc et al., 2012), flocculation phenomena which affect froth stability (Farrokhpay and Bradshaw, 2012), and slimes coating phenomena (Tao et al., 2010). Chlorite is notably present in many base metal sulphide and oxide flotation systems and its removal is critical to achieving concentrate grades which are acceptable to further processing (Silvester et al., 2013). Another problematic phyllosilicate is talc which, due to its natural hydrophobicity, readily reports to the concentrate, increasing the viscosity in the froth phase and resulting in a reduction in both grade and recovery (Becker et al., 2009). Talc also reduces the density in leaching circuits, affecting throughput and recovery (Macfarlane and Williams, 2014). Large quantities of talc in the concentrate can cause problems during smelting, often resulting in penalties for mineral processing companies (Beattie et al., 2006).
Leaching is equally impacted by the presence of phyllosilicate minerals because these hinder percolation and increase viscosity due to their characteristically small particle size (Farrokhpay and Bradshaw, 2012, Tremolada et al., 2010). This may lead to overflowing tanks as a result of a reduced rate of discharge from the tank. Chlorite is known to be a powerful long-term acid consumer (Jansen and Taylor, 2003). In acidic solutions, under ‘ideal’ stoichiometric conditions, chlorite dissolves according to following reaction (Lowson et al., 2005):(Mg, Fe, Al)6[AlSi3O10](OH)8 + 16H+ ⇋ [6(Mg, Fe, Al)]13+ + Al3+ + 3H4SiO4 + 6H2O
Compared to chlorite, biotite consumes acid more rapidly at low pH and is more reactive towards strong acids (Free, 2010, Jansen and Taylor, 2003). Previously, it was shown that intra-deposit variation in modal percentages of magnesium silicate minerals led to different flotation characteristics for the same valuable minerals (Lotter et al., 2003). Clay minerals, particularly those of the smectite group, have the potential to retain acid in their structures (Chetty and Deshenthree, 2018). Although acid is adsorbed rather than converted, it is rendered unavailable for reaction with target minerals.
Processing strategies to remove clay-like slimes include de-sliming (Filippov et al., 2016, De Cuyper, 1988), a (talc) pre-flotation stage (Formanek and Lauvernier, 1963, Lutandula and Maloba, 2013, Shengo et al., 2019), or depression during flotation (Bulatovic, 2010). Depression of chlorite in oxide and sulphide flotation can be achieved with reagents typically used for the depression of silicate minerals: sodium silicate (Na2SiO3), carboxymethylcellulose (CMC) and fluorocompounds (Silvester et al., 2013).
4.1.2.4. Sulphide gangue (pyrite and pyrrhotite)
Pyrite is the most abundant sulphide gangue mineral in all cobalt ores and it may contain significant amounts of cobalt in the form of cobaltiferous pyrite (De Cuyper, 1981). When present as a gangue mineral, it is problematic in terms of its impact on selective flotation of cobalt minerals (Barbery, 1986). Pyrite is readily oxidised under atmospheric conditions, which often leads to the presence of elemental sulphur on the mineral surface. While dependent on the crystallinity of the sulphur coating, pyrite can be highly floatable and difficult to separate from cobalt-bearing minerals. Depression of pyrite may be achieved in alkaline conditions (pH > 11.5) through the addition of organic compounds, sulphates and cyanide (Bulatovic, 2007a). The use of sodium sulphide during the flotation of cobaltite with sodium diethyl dithiophosphate is reported to depress pyrite (Abeidu, 1976).
Pyrrhotite is a common gangue mineral in magmatic Ni sulphides deposits, occurring in monoclinic and hexagonal forms. The crystal structure plays a critical role because monoclinic pyrrhotite is ferromagnetic, while the hexagonal form is not. Monoclinic pyrrhotite can be partly rejected by magnetic separation while hexagonal pyrrhotite is usually separated by flotation (Bulatovic, 2007b). The latter may be problematic when intergrown inclusions of pentlandite or nickel in solid solution are present (Rao, 2000). Rejecting hexagonal pyrrhotite may be achieved by (i) staged flotation with timings based on differences in flotation rate, which are relatively low for pyrrhotite, (ii) floating in a highly alkaline solution with cyanide as depressant and xanthates or dithiophosphates as collectors (Rao, 2000), (iii) application of triethylenetetramine and sodium metabisulphite (Kelebek and Tukel, 1999), or (iv) reverse flotation of the pyrrhotite before pentlandite flotation (Moyer, 1948).
Iron sulphides oxidation, which can be very rapid in bio-heap leaching in the absence of biotic mediation, is known to be the major source of the self-heating phenomenon (Ghorbani et al., 2016). While it can be improve the overall leaching efficiency, as higher temperatures enhance reaction kinetics, it can also lead to auto-ignition in the presence of combustible material in the heap (Rosenblum and Spira, 1995).