1. Introduction
The effluent produced by companies contains a variety of organic chemicals, with dyes being one of the main contaminants. The dyes in the wastewater have been causing major environmental problems for a number of years. There are many companies that process materials using dyes, and it is estimated that 10 to 15% of the dye applied for the process is lost in the effluent during the dying process [1,2]. A million tons of dyes are generated annually, and 50% of these dyes are used in the textile industry, according to data [3]. One of the difficult issues for pursuing humankind's sustainable growth is the removal of synthetic dyes from various industrial effluents [4]. For example, disperse, acidic, basic, sulfur, reactive, azo, indigoid, diazo, triphenylmethyl, phthalocyanine, metal-complex dyes and anthraquinone, in addition to derivatives of the aforementioned, are just a few of the structural and chemical variations that exist for synthetic dyes. The colors' intricate structure and synthetic origin make cost-effective, extensive treatment of the dyeing effluent an unquestionably challenging challenge to solve. In order to test their ability to remove dye, a variety of techniques have been suggested. Examples include biological methods, physical/chemical routes, chemical decomposition, enzymatic decomposition, and electrochemical processes, as well as advanced oxidation processes, membrane separation, adsorption, and other traditional wastewater treatment techniques [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14]]. One of these techniques, semiconductor photocatalysis, is believed to be an effective way to use solar energy to degrade organic dyes. The technology is economical and environmentally benign since it converts large-scale organic contaminants into low or non-toxic tiny molecules by mineralization.
In recent years, semiconductor photocatalysis has advanced significantly [[15], [16], [17]]. Research on photocatalysis has centered on TiO2 in particular because of its abundance in nature, chemical stability, and lack of toxicity [18,19]. However, this material's high band gap (3.2 eV), which only allows for the absorption of UV light, is a drawback [20]. In order to increase the photocatalytic efficiency of TiO2 by using a larger portion of the solar light for photocatalysis, the absorption properties of TiO2 have been extended to the visible region of the spectrum. This is because only 3% of the solar radiation on earth is UV radiation [[21], [22], [23]]. One of the most researched photocatalysts is titanium dioxide (TiO2), which has demonstrated outstanding potential for use in environmental applications [24]. TiO2 has previously been investigated for use as a photocatalyst to purify water and wastewater, demonstrating its potential in this field of photocatalytic treatment [25,26]. Due to TiO2 having high band gap (3.2 eV) and quick recombination of photoexcited electrons/holes, which limits the photocatalytic capacity of TiO2, the efficiency of TiO2 as a photocatalyst is insufficient within the solar spectrum [27,28]. In recent decades, research has focused on methods to get limit of TiO2. The creation of TiO2 composites permits the amendment of morphology and fine-tuning of properties for better performance. Because of their inherent chemical and mechanical properties, noble metals, GO, metal-organic frameworks, semiconductors and carbon nanotubes hold significant promise in the simple create of efficient TiO2 hybrid nanocomposites [[29], [30], [31], [32], [33]]. Moreover, owing to the electric mobility and high specific areas of carbon nanomaterials, which are advantageous to TiO2 based nanocomposites, the noble metal introduces plasmonic and Schottky effects [34,35]. For that reason, the construction of TiO2 based nanocomposites with GO, noble metals, carbon nanotubes, semiconductors and metal organic frameworks hinder the recombination of photogenerated electron-hole pairs, consequently improving the transport of the photoexcited electrons to the interface between the aqueous media and photocatalysts, enhancing the effectiveness of photocatalysis [36].
Various specialized aspects of TiO2 photocatalysis have been examined individually over the past few years. This research examined recent developments in the mechanistic analysis, practical use and significantly improved TiO2 based heterostructure photocatalysts. Firstly, the basic principles of photocatalysts are discussed. Secondly, a number of techniques for improving the behavior of this hybrid nanocomposite in terms of different parameters, such as conductive medium, pH, co-catalyst, and architecture are described. Thirdly, the development of TiO2 based hybrid photocatalysts have explored for the photodegradation of organic dyes and the promotion of a method for treating organic wastewater. Finally, the difficulties and prospects for further study on these photocatalysts are discussed.
2. Basic principle of photocatalysis
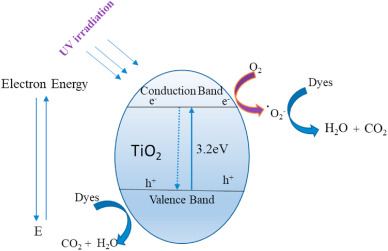
An effective and low-impact technique for removing organic pollutants and non-biodegradable compounds from wastewater is photocatalysis. This technique has been demonstrated to effectively remove a variety of organic contaminants from water and wastewater, including herbicides, humic acid, pesticides, pharmaceuticals and halophenols [[37], [38], [39], [40], [41], [42], [43], [44], [45]]. The fundamental principle of dyes conversion on a photocatalyst is demonstrated in Fig. 1. The semiconductor absorbs energy during photocatalysis that is equal to or more than the band gap energy, which causes the electron to be stimulated from the valence band (VB) to the empty conduction (CB) of the photocatalyst. Afterward, the VB obtains a positively charged hole and it is oxidizing whereas the CB obtains a negatively charged electron and it is reducing, as demonstrated in Eq (1).(1)(2)(3)(4)(5)
 Fig. 1. The possible reaction mechanism of dyes using TiO2 photocatalyst.
Fig. 1. The possible reaction mechanism of dyes using TiO2 photocatalyst.The main benefit of heterogeneous photocatalysis is that it can take place in ambient conditions, where the atmospheric oxygen is sufficient as oxidant. After production of •OH radicals, the organic pollutant can be photodegraded (Eqs (2), (3), (4), (5))). In addition, through this procedure, all organic compounds are completely mineralized into H2O and CO2 [[46], [47], [48], [49], [50], [51], [52]]. Due to their broad surface area and efficient photocatalysis, TiO2 nanoparticles have attracted a lot of attention recently [[53], [54], [55]]. Rutile (tetragonal), anatase (tetragonal) and brookite (orthorhombic) are the three crystalline phases of TiO2, which has a band gap of about 3.2 eV [[56], [57], [58], [59], [60]]. The physicochemical characteristics of TiO2, such as its crystal structure, impurities, surface area, surface morphology, hydroxyl group densities at the active sites, thickness and porosity, all affect its photocatalytic activity [[61], [62], [63], [64]]. On the other hand, it can be stabilized on different supports by a number of techniques [[65], [66], [67], [68], [69]].
3. Synthesis of TiO2 based nanocomposites
3.1. Sol gel technique
Typically used in the manufacturing of nanoparticles, the sol-gel process is a simple low-temperature method. It provides various benefits, including excellent homogeneity, a quick process and a low calcination temperature and duration. Therefore, the nanoparticles gained high crystallinity and larger surface area. The precursors of the materials acquired through this method were dissolved in a solvent while the pH was managed until the sol was created [70]. Eventually, the gel was calcined at the optimum calcination temperature to create nanoparticles. For instance, Hasan et al. studied the TiO2/GO/Cr2S3composite was manufactured by adding GO and TiO2 by sol gel technique, as depicted ion Fig. 2 [71]. Characteristically, after dissolving 0.1 mol of Cr(NO3)3·9H2O in 30 mL of deionized water, 0.1 mol of Na2S·9H2O was added. Hereafter, 25 mL of ethanol was applied to dissolve 500 mg of graphene oxide, which was then agitated constantly for 1 h. The abovementioned solution was mixed with the dispersed graphene oxide, which was then added, and the mixture was constantly agitated at 70 °C for 5 h. Filtration, washing, and drying at 100 °C for 24 h followed by the solution. The composites of Cr2S3 and GO were made after drying. The reaction mixture given above was modified with TiO2SO4·H2O (0.1, 0.3 mol), and the end result were continuously agitated at 70 °C for 5 h. The resulting Cr2S3-modified TiO2 followed filtering, washing, and 3 h of drying at 100 °C. The TiO2/GO/Cr2S3 nanocomposite was produced by further heating the obtained product at 500 °C. Marien et al. also evaluated a simplistic technique to tune TiO2 NPs morphology by amending and an acid-catalyzed sol-gel preparation with Pluronic P123 [72].
 Fig. 2. Manufacturing process of Cr2S3-GO/TiO2 composites [Reprinted from ref. 71].
Fig. 2. Manufacturing process of Cr2S3-GO/TiO2 composites [Reprinted from ref. 71].3.2. Solvothermal/hydrothermal method
Inorganic chemistry generally uses a solvothermal or hydrothermal approach to create inorganic nanomaterials with different morphologies for various uses. These two strategies differ in that the former utilized water as a solvent. In this approach, solvents are found in autoclave vessels made of sealed steel autoclave, where a chemical reaction is carried out under specified temperature and pressure. The formation of crystals requires two steps: crystal nucleation and crystal growth. In addition, this system precisely controls a wide range of synthesis-affecting variables, including additives, pH, temperature and others [73]. For example, Nguyen et al. reported that the TiO2/ZnO/rGO composite has been constructed via a hydrothermal procedure exploiting GO, Zn(CH3OO)2 and TiF4 as the precursors [74]. Consequently, an appropriate amount of GO (1%, 3%, 5%, or 10% by weight) was ultrasonically processed in 45 mL of ethanol for 30 min to thoroughly exfoliate the GO and designated as solution A. To produce an uniform mixture, 45 mL of deionized water was mixed with the required amounts of Zn(CH3OO)2 and TiF4 (1:1), then stirred continuously and sonicated for 15 min in each procedure (solution B). Afterward, adding solution A to solution B, they were magnetically agitated for 1 h. Drop by drop, NH4OH has been mixed to the suspension to adjust the pH to 11. After that, the resulting suspension was put into a Teflon-lined autoclave for 20 h at a regulated temperature of 180 °C. In hydrothermal environments, ethanol in water behaved as a reductant to transform GO into rGO. To get removal of the impurities, the precipitate was washed several times with deionized water and ethanol. The resulting solid was calcined at 300 °C for 30 min after being dried in a vacuum oven at 70 °C overnight. As well as, Hao and co-workers reported an effective TiO2/MXene/NiFeCo composite that were successfully build by a facile solvothermal method [75]. Tian et al. created the new TiO2/UiO-66-NH2/Ti3C2 composite was logically manufactured by introduced Ti3C2TxMXenes on water stable Zr-MOFs precursors through a facile hydrothermal procedure, as shown in Fig. 3 [76].
 Fig. 3. Representation illustration demonstrating probable reaction direction for the development of TiO2/Ti3C2/UiO-66-NH2 [Reprinted from ref. 76].
Fig. 3. Representation illustration demonstrating probable reaction direction for the development of TiO2/Ti3C2/UiO-66-NH2 [Reprinted from ref. 76].3.3. Microwave assisted technique
Because of its high rate of reaction, simplicity and efficiency, the microwave supported technique is becoming more and well-liked in the production of nanoparticles. Using this procedure, materials were heated by irradiation rather than thermal heating, which was utilized in conventional procedures. Microwave irradiation's interaction with the polar molecule produced a dipole moment in the reaction mixture. The rotation of the molecule may be influenced by the activity [77,78]. This orientation increases the probability of molecular collisions while lowering energy activation and heat production, which leads to an increase in reaction and the manufacturing of the nanoparticle. Kubiak et al. reported the synthesis of the TiO2/CuO composite contained of two steps [79]. Initially, 100 mL of 10% titanium (IV) chloride was put in a reactor on a magnetic stirrer after a 10% aqueous solution of TiCl4 was manufactured. After that, a 5 M solution of NaOH was mixed at a dosage rate of 5 mL/min until the pH was 10. After that, the reaction mixture was moved to an SP-D80 microwave reactor and heated for 10 min at 200 °C using a 300 W power source. The produced TiO2 was filtered, rinsed 3 times with distilled water and then dried for 12 h at 105 °C. Then, 2 g of poly (ethylene glycol) (PEG) have been mixed to 25 mL of a copper (II) acetate aqueous solution at a concentration of 5%. The addition of poly ethylene glycol was applied to enhance the bond between CuO and TiO2. When the pH reached 12, 1 M NaOH solution was mixed to the synthesized solution in a reactor on a magnetic stirrer. Titania that had been made in the first step was sonicated in water for 10 min before being mixed to the action mixture and stirring for 30 min. The final step was treating the resultant solution with a 300 W microwave at 150 °C for 10 min. The as-prepared composite was filtered, cleaned with distilled water three times and dried at 70 °C for 7 h.
3.4. Photoreduction methods
Another interesting technique for producing noble metal-TiO2 composites is photoreduction, which includes in situ reduction of metal ions adsorbed on TiO2 surface. A noble metal precursor, supporting TiO2 nanostructures like nanoparticles synthesized through hydrothermal or sol-gel methods, and photoirradiation are all present during the synthesis [80,81]. The noble metal nanostructures thus created on TiO2 nanoparticles were efficient of being completely coupled to the TiO2 surface with sharp interfaces forming Schottky barriers, which are helpful for trapping photoexcited electron and then charge transfer mechanisms to occur, adding to the multifunctional nature of the prepared nanocomposite [82]. Applying this production method, Kowalska and his group manufactured TiO2 tailored with photodeposited gold nanoparticles, which demonstrated superior photocatalytic characteristic under visible light illumination [83].
4. Photocatalytic degradation of organic dye compounds by using TiO2 based composites
4.1. Semiconductors supported TiO2 based composites
Due to its physicochemical and enduring photo-stability, in addition to its affordability and ease of manufacture, titanium dioxide (TiO2) was encouraged as a tremendous and expansively utilized semiconductor samples for the photodegradation of important organic pollutants [[84], [85], [86], [87]]. TiO2has been used in the majority of photocatalytic applications despite having a number of disadvantages, but suffers from its ease of photogenerated charge carrier's recombination and energy absorption at the UV portion of the solar spectrum [88]. Several researches have focused on two primary directives to increase the TiO2 photocatalytic activity: (1) lower the photoexcited electron-hole recombination rate and increase its practical operating range and (2) increase its surface region and improve the adsorption characteristics [[89], [90], [91], [92], [93], [94]]. Hitherto, recent studies have indicated that combining with other semiconductors, such as SnO2, NiO, Bi2O3, SbxSn1-xO2, FeTiO3, In2O3, ZnO, CdS, ZrO2, CuS, Fe2O3, WO3, SiO2, and others, can greatly enhance the photocatalytic activity [[95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110]]. What is more, the VBof WO3 has the potential to excite and transfer electrons to CB by absorbing visual energy from the solar spectrum. As well, WO3 performs as a hunter for electrons that are photogenerated and attained from TiO2 because it has appropriate CB and VB positions with regard to TiO2. In the area of the VB of TiO2, holes produced by photoexcited of the WO3 VB transfer and accumulate in the valence band (VB) [111,112]. This approach enhances the capability for charge transfer, which in turn enhances the photoactivity of the WO3/TiO2composite.
Recently, Du et al. constructed the core shell structured TiO2/In2O3with high visible-light photoactivity was created by solvothermal technique [113]. With a direct band gap of 3.6 eV and an indirect band gap of 2.8 eV, In2O3 is an indirect band semiconductor. It may successfully transfer the absorption band edge of oxide semiconductor photocatalysts from the UV area into the visible region, making it an important sensitizer. Besides, in the case of suitable conduction band potentials, the integration of TiO2 and In2O3 could transfer from an excited small band gap semiconductor into another attached one, achieving efficient separation of photoexcited electron/hole pairs and considerable value the photoactivity of semiconductor heterostructure. Furthermore, in comparison to pure TiO2 and In2O3 products, the developed In2O3/TiO2 with core-shell structure indicated is very photocatalytic activity to reduce MO.
lately, Sandra et al. reported the phosphorous doped TiO2/α-Fe2O3photocatalysts has invented by the microwave-assisted sol-gel technique [114]. P–TiO2/α-Fe2O3 was analyzed as a sulfonamide mixture photodegraded under visible light. A face-centered central composite design was utilized to optimize the photocatalytic process. Due to substitution doping, P doping shifted the α-Fe2O3/P–TiO2 catalyst's light absorption in the visible light range, however coupling P–TiO2 with α-Fe2O3 increased the absorption in the visible range. This increased the charge carrier lifetime and gave the α-Fe2O3/P–TiO2 catalyst greater photoactivity to TiO2. Using the P–TiO2/α-Fe2O3/PS system, the biodegradability index was 0.48, demonstrating a less hazardous effluent than the original components. During the photodegradation of the sulfonamide through the coupled process, two significant intermediates (pyrimidine and hydroquinone) and their hydroxylated re-arrangements have been noticed. As a result of the SNs' degradation, sulfonic, oxyalic, oxyamic and acetic acids were also recognized as by-products.
Furthermore, TiO2/BiOX (X = Cl, Br, and I) hierarchical nanocomposites with remarkable performances were effectively created by a one-pot, low-temperature solvothermal procedure without the use of any structure-directing chemicals, according to Yao et al. [115]. The rapid photodegradation of colored and colorless organic pollutants effectively illustrated the dual capabilities of adsorption associated with photocatalysis based on TiO2/BiOX. Particularly, the optimized BiOCl/TiO2 nanocomposites were found to have much higher adsorption and photocatalytic efficiency than their competitors. The presence of columbite TiO2-II in anatase, which not only enhances the ability to absorb visible light and the charge separation efficiency but also allows for efficient customization of particle size, surface area and exposed active facet of composites, should be credited for the development of adsorption and photocatalytic performances.
Another study, Sheng et al. studied the 3D TiO2/g-C3N4 heterostructure was prepared and shows capable contaminant photodegradation performances [116]. The type II heterojunction between g-C3N4 and TiO2, as seen in Fig. 4(a, b), is produced and efficiently inhibits the recombination of photo-generated charge carriers. Since superoxide radicals play an important part in the photocatalytic activity, the photogenerated electrons on the CB of g-C3N4 can easily be moved to TiO2 and subsequently combine with the dissolved oxygen to produce them. Both radicals and holes have a powerful capability for oxidative degradation and perform as the reaction's active species. In a static environment, the photodegradation efficiency of methylene blue (MB) and phenol are 4.0- and 4.5-folds superior to those of bulk g-C3N4, respectively. With a removal rate of 16.0% in a dynamic system, the pollutant can be constantly degraded without separation and is highly stable for 90 h. The g-C3N4/TiO2 composite 3D structure and structure is primarily responsible for the greater activity. The 3D structure gives multidimensional mass and electron transfer pathways in addition to greatly improving the adsorption-enrichment capability. In the meantime, the heterostructure can encourage the partition and migration of the photoexcited charge carriers. Consequently, the 3D g-C3N4/TiO2 heterostructure with partition free can assist the potential utilizations in the handling of water contamination.
 Fig. 4. (a) Schematic design of photogenerated electron/hole pair partition by TiO2/g-C3N4 (b) Schematic diagram of the feasible photodegradation mechanism [Reprinted from ref. 116].
Fig. 4. (a) Schematic design of photogenerated electron/hole pair partition by TiO2/g-C3N4 (b) Schematic diagram of the feasible photodegradation mechanism [Reprinted from ref. 116].Masudur et al. produced the composite TiO2/WO3 working in visible-light was synthesized using cis-butenedioic acid to combine component semiconductors of WO3 and TiO2 NPs [117]. In order to degrade phenol and methylene blue dye in aqueous under visible light, the composite's photocatalytic activity was tuned to 5 mol% TiO2/WO3. After that, the surface of the enhanced TiO2/WO3 was covered with Au NPs. Under visible light, the photoactivity of TiO2/Au-WO3 was revealed to photodegrade above organic contaminants in aqueous solution (depicted in Fig. 5). In the VB of WO3 during photoexcitation in the presence of visible radiation, h+ are produced. Since the VB of TiO2 is situated above the VB of WO3, these photoexcited h+ are likely to move to the VB of TiO2 NPs. Nevertheless, recombination of photogenerated e‾ and h+ will be efficiently suppressed owing to the presence of Au NPs on to the surface of WO3/TiO2nanocomposite. Therefore, the h+ produced at the valence band of TiO2 will take part at dissimilar oxidation reactions with adsorbed organic pollutants and/or with H2O/OH‾ to form •OH to degrade organic pollutants. When compared to a 5 mol% WO3–TiO2 composite, TiO2/Au-WO3 showed extremely high photoactivity in the degradation of the pollutant. After 120 min of visual irradiation, 52% photodegradation of MB dye and 63% degradation of phenol were observed under the same conditions. Au-WO3/TiO2 composite's significant rise in photocatalytic performance is ascribed to the good energy band matching between WO3 and TiO2, which inhibits photogenerated charge carriers recombination and encourages particle-to-particle transfer between the two materials. Au NPs increase transport of e‾ from the conduction band of WO3. Therefore, possible recombination of photogenerated charge pair was hindered and photocatalytic effectiveness was significantly enhanced [118].
 Fig. 5. Schematic illustration for photocatalysis with Au doped WO3/TiO2heterostructure in visible light [Reprinted from ref. 117].
Fig. 5. Schematic illustration for photocatalysis with Au doped WO3/TiO2heterostructure in visible light [Reprinted from ref. 117].The magnetically separable n-p junction TiO2/Fe3O4/BiOI composites, which are thought to be unique visible light photocatalysts, were created by Gholamian et al. using a simple process for the decontamination of various dyes, as demonstrated in Fig. 6 (a, b) [119].
 Fig. 6(a). The Schematic drawing for BiOI/TiO2/Fe3O4 photocatalyst synthesis (b) A probable mechanism of photocatalytic reaction over the BiOI/TiO2/Fe3O4photocatalyst via n–p heterostructure [Reproduced from ref. 119].
Fig. 6(a). The Schematic drawing for BiOI/TiO2/Fe3O4 photocatalyst synthesis (b) A probable mechanism of photocatalytic reaction over the BiOI/TiO2/Fe3O4photocatalyst via n–p heterostructure [Reproduced from ref. 119].The Fe3O4/TiO2/BiOI (20%) sample, which was around 6.85 and 4.39 times superior to the TiO2 and TiO2/Fe3O4 material, was found to have the best photodegradation activity for RhB. The enhanced specific surface area and the development of n-p heterojunctions between TiO2 and BiOI semiconductors could be attributed to the rise in photoactivity. Excellent activity was also shown in the degradation of the dye's methyl orange, fuchsine, and malachite green by the TiO2/Fe3O4/BiOI 20% composite. The findings show that O2•- was essential for the photocatalytic removal of RhB. In addition, the TiO2/Fe3O4/BiOI 20% composite illustrated more photocatalytic activity. The current work offers a novel method for producing TiO2-based ternary visible light photocatalysts with enhanced performance for water filtration. It holds considerable promise that the making of heterojunction photocatalyst will enable visible light activation and prevent photogenerated charge pair recombination.
In this, Huy et al. generated the TNT (TiO2 nanotube)/SnO2 composite through a single step hydrothermal approach and thoroughly examined NO photodegradation over the TNT/SnO2 composite in visible light [120]. Fig. 7., depict the photocatalytic mechanism of the TNTs/SnO2 for NO photodegradation.
 Fig. 7. NO photodegradation mechanism of the TNTs/SnO2 heterostructure [Reproduced from ref. 120].
Fig. 7. NO photodegradation mechanism of the TNTs/SnO2 heterostructure [Reproduced from ref. 120].In that, the generation of electrons and holes in the material's VB and subsequent electron transport to the material's CB occur when the SnO2/TNTs composite is activated. When these electrons reduce adsorbed O2 or oxygen gas, they can either move to the surface of the TNTs or migrate to the conduction band of SnO2 nanoparticles, where they will then produce superoxide radical anions. Likewise, these electrons can either travel to the surface of the TNTs or transfer to the conduction band of SnO2 nanoparticles, where they will then make superoxide radical anions, when they decrease adsorbed O2 or oxygen gas. Furthermore, the main active species that are essential in the NO photocatalytic degradation are •O2− and •OH.
For the direct Z-scheme system, two suitable photocatalysts are in close contact without an electron/hole migrate moderator. More importantly, for the direct Z-scheme system, the backward reactions and light shielding effect happening on traditional and all-solid-state Z-scheme systems can be avoided because of the nonattendance of the redox moderator. A typical case is that the Z-scheme TiO2/g-C3N4 nanotube array displayed improved photocatalytic activity in visible light illumination [121]. Based on the systematically characterizations, it is confirmed that the photoexcited e− in the CB of TiO2 are recombined with the holes in the VB of g-C3N4 during the photocatalytic reaction, while the photogenerated electrons in the conduction band of g-C3N4 and holes in the valence band of TiO2 with strong redox ability are involved in dye degradation, as shown in Fig. 8.
 Fig. 8. Representation drawing of the charge transfer and partition on Z-scheme TiO2/g-C3N4 composite in visible light irradiation [Reproduced from ref. 121].
Fig. 8. Representation drawing of the charge transfer and partition on Z-scheme TiO2/g-C3N4 composite in visible light irradiation [Reproduced from ref. 121].It would be ideal to develop photocatalytic systems with a larger design to degrade dye contaminants using solar light. A binary mixture of MB and Rhodamine B was simultaneously degraded using a S-scheme (step-scheme) TiO2/g-C3N4 composite that was created by Barzegar and his group [122]. The as-prepared composite exhibits greater photocatalytic activity than pure TiO2and g-C3N4, which is attributable to the beneficial synergistic impact of the S-scheme between g-C3N4 and TiO2 nanostructure. In PTC, TiO2/g-C3N4 was able to degrade the binary mixtures MB and RhB by around 94.92 and 93.07%, respectively. The excited electron on the surface of g-C3N4 can be transported to TiO2 via the well-designed heterojunction because the CB edge potential of g-C3N4 is more negative than TiO2. The charge transfer mechanism of the S-scheme heterojunction between n-type g-C3N4 and n-type TiO2 is depicted in Fig. 9 (a, b). The radical trapping tests have demonstrated that this electron transfer pathway results in outstanding redox capacity [123]. The solid-solid heterojunction interface particles produced by the strong coupling of TiO2 and g-C3N4 improve photo-generated charge carriers and boost photocatalytic activity. As a result of the photo-induced holes' tendency to move from the CB of TiO2 to the valence band of g-C3N4 when exposed to solar radiation, an S-scheme photocatalytic system is formed. The valence band holes in TiO2 are located lower than the OH−/˙OH, which has the potential to oxidize water to generate ˙OH. Next, O2 reacts with the electrons that g-CB C3N4 has stored to create an active superoxide radical ion (˙O2−). These extremely aggressive radical species may eventually breakdown the dyes into carbon dioxide and water [124].
 Fig. 9. (a and b) Representation diagram of the suggested mechanism for TiO2/g-C3N4 solar light irradiation [Reproduced from ref. 122].
Fig. 9. (a and b) Representation diagram of the suggested mechanism for TiO2/g-C3N4 solar light irradiation [Reproduced from ref. 122].4.2. GO supported TiO2 based nanocomposites
Six carbon atoms, formed by the sp2 hybridization of carbon atoms, form a flat hexagonal lattice with honeycomb architecture in graphene, a material with distinctive electronic properties [[125], [126], [127], [128], [129], [130], [131], [132], [133], [134], [135]]. Currently, a single sheet or many layers of graphene are generated using a wide range of procedures. Due to its low cost and simplicity of use, the chemical exfoliation of graphite by the introduction of oxygen functional groups and graphene oxide (GO) development is known as the most prevalent approach. Here in, Nguyen et al. produced the TiO2/rGO/ZnO composites have been manufactured via a simplistic hydrothermal procedure [74]. The efficiency of these composite photocatalysts was then estimated by looking at the removal of MB, Rh B and MO from water under UV/simulated solar irradiation. Compared with ZnO, TiO2 and TiO2/ZnO, the synergism of TiO2/rGO/ZnO nanocomposites confirmed greater photoactivity. After 120 min of UV exposure, MB over TiO2/rGO/ZnO showed the maximum photodegradation and mineralization, at 99.6% and 59.0%, respectively. After 180 min of UV irradiation, these efficiencies also delivered 99.2% and 48.0% for Rh B and 99.4% and 44.2% for methyl orange.
Lately, Hasan et al. evaluated that the ternary TiO2/Cr2S3/GO photocatalyst has been manufactured through coupling GO and TiO2 by sol-gel method [71]. The TiO2/Cr2S3/GO composite's photoactivity was schematically represented in Fig. 10.
 Fig. 10. Representation illustration of the photoactivity of the GO/TiO2/Cr2S3photocatalyst [Reproduced from ref. 71].
Fig. 10. Representation illustration of the photoactivity of the GO/TiO2/Cr2S3photocatalyst [Reproduced from ref. 71].The catalyst is activated and produces electron/hole pairs when light strikes it. These electrons combined with the O2 atoms in the area and result in the production of superoxide radicals •O2−. Moreover, light that is absorbed by the surface of graphene oxide can readily produce more electrons, in addition to more superoxide ions and hydroxyl radicals. The fragmentation of dyes into CO2and H2O occurs as a result of the creation of (OH−) and (•OH). The as-prepared nanocomposite showed improved photocatalytic activity and photodegradation rate yielded 98.3, 96.6 and 86.3% on MB, Rh B and MO, respectively, for 120 min under the visible light irradiation, which is greater than that attained by GO/Cr2S3 and TiO2 photocatalyst. As evidence showing the alteration of Cr2S3 on TiO2 can increase TiO2 photoactivity, reducing the detected contaminants for a non-pollutant, Cr2S3 and GO play a significant role in the photoactivity of TiO2. To create a water environment free of contaminants, this may aid in the degradation of pollutants.
More recently, the green alga Chlorella pyrenoidosa was used in the current study to describe an innovative and environmentally acceptable process of producing TiO2 nanoparticles. The development mechanism of TiO2 NPs, GO and TiO2/GO composite is revealed in Fig. 11(a). To create a TiO2/GO composite, these TiO2 NPs were also placed on GO nanosheets [136]. The model pollutant utilized was the Crystal Violet dye and photocatalytic mechanism was shown in Fig. 11(b). The photodegradation of Crystal Violet dye in an aqueous mediaunder visible light was used to assess the photocatalytic activity of the TiO2 NPs and TiO2/GO composite. The photogenerated electrons react with O2 and produce superoxide radicals and consequently, the hole in the VB reacts with H2O and makes hydroxyl radicals. These reactive oxygen groups oxidize CV dye to create CO2, H2O and intermediates. In comparison to pure TiO2 NPs, the TiO2/GO composite demonstrated greater photocatalytic efficiency and three cycles of reusability. The enhanced charge separation, efficient charge transportation, increased dye adsorption, and expanded light absorption range may all be accountable for the TiO2/GO composite's higher activity. This composite may be used to purify the water and has tremendous potential to remove environmental contaminants.
 Fig. 11. (a) Synthesis steps for (i)TiO2 nanoparticles from green alga Chlorella pyrenoidosa, (ii) GO using modified Hummer's process and (iii) TiO2/GO sample utilizing hydrothermal procedure (b) Proposed mechanism for photodegradation of CV dye employing TiO2/GO photocatalyst in visible light [Reproduced from ref.136].
Fig. 11. (a) Synthesis steps for (i)TiO2 nanoparticles from green alga Chlorella pyrenoidosa, (ii) GO using modified Hummer's process and (iii) TiO2/GO sample utilizing hydrothermal procedure (b) Proposed mechanism for photodegradation of CV dye employing TiO2/GO photocatalyst in visible light [Reproduced from ref.136].On the other hand, Arham et al. fabricated the elimination of acid navy blue utilizing TiO2/GO composite, which is a next generation photocatalyst because of its attractive optical features [137]. Pristine TiO2 NPs were prepared by the hydrolysis of Ti{OCH(CH₃)₂}₄ (Titanium tetra isopropoxide) while GO synthesized by enhanced Hummer's process. GO and TiO2 suspensions were ultrasonically mixed to make GO/TiO2 composites with various GO concentrations. The photodegradation of acid navy blue dye was used to investigate the photocatalytic abilities of the nanocomposites, as depicted in Fig. 12.
 Fig. 12. Representation illustration for photocatalytic mechanism via GO/TiO2photocatalyst [Reproduced from ref. 137].
Fig. 12. Representation illustration for photocatalytic mechanism via GO/TiO2photocatalyst [Reproduced from ref. 137].