Introduction
Protein-based natural polymers (e.g., collagen, fibroin, gelatin, albumin, etc.), polynucleotides; Deoxyribonucleic acid (DNA), Ribonucleic acid (RNA), and polysaccharides (e.g., chitosan, chitin, cellulose, hyaluronic acid, dextrin, etc.) are being widely used in medical applications due to having some intrinsic properties of drug delivery, excellent bioactivity, biodegradability, hemostatic effect, antimicrobial, antihypertensive, antioxidant and low antigenicity just because of easy surface medication advantage [1], [2], [3], [4], [5], [6], [7]Moreover, bio-degradable collagen exhibits high water affinity, good cell compatibility (adhesion, growth, migration), structural integrity, cellular affinity, and activated platelets for wound tissue proliferation [8], [9], [10].
Among biopolymers, collagen is a structural protein to be one of the most suitable artificial film constituents for damaged tissue regeneration[11,12].Collagen is a fibrous structural protein found in the animal kingdom but not in plants, and the term is derived from the Greek word (kola means gum and gen means producing) [13]. When compared to the cell response-ability within the other variants, the collagen template is a sufficiently good fit for bio-mineralization which acts as a building block for connective tissues such as bone, cartilage, tendon, and so on [14]. The process of biomineralization involves the integration of organic and inorganic materials under the control of living organisms. Having the properties, human-like collagen (HLC) becomes a primary material for skin grafts, artificial bones, blood vessels, etc. [15]. However, when cells are cultured in vitro, it is one of the most common ECM (responsible for the physical maintenance of protein cells) components that hold the entire body together and provide an adequate environment for the synthesis or assembly of new fibrils [9]. On the total weight of body cells, approximately 30 % of the weight is comprised of a plethora of collagenous protein [14,16]. Owing to the protein abundance, this offers tissue growth dramatically to internal and external organs (e.g., dermis, bone, cartilage, tendon, ligament,). L. Joseph et al. invented a body absorbable surgical suture named “catgut” in 1881 for use in biomedical areas [17]. Every year, people get affected by diseases or by injury by burns or any other means, so need to artificial membrane because the donor site is very incommensurate with the demands, and also a varying cell from man to man has an adverse immunology response. Collagen has a quick biodegradation characteristic but non-sterilized collagen in biomedical applications promotes highly infectious diseases [18,19]. ECM is a non-cellular 3-dimensional component found in all human tissues and organs that are made up of collagens, elastin, and a variety of other proteins that determine biochemical and biomechanical properties. Collagen, one of the main structural component of the ECM, provide tensile strength, regulate cell adhesion and migration, and guide tissue development [20]. It is increasingly being used in combination with other polymers for the fabrication of fascinated materials, such as chitosan, elastin, fibroin, polycaprolactone (PCL), and polyethylene oxide (PEO) for wound dressing mats, artificial skin, hydrogels, etc. [21], [22], [23].
Several methods, such as film casting, situ preparation, electrospinning, layer-by-layer assembly, ionotropic gelation, covalent coupling, sol-gel technique, freeze-drying, etc., have been used to produce nanocomposite materials using collagen protein. The designed manufacturing materials are now also being fabricated using computer-aided manufacturing-based rapid prototyping (RP) methods [24]. However, one of the most significant challenges for native collagen-based biomaterials for medical applications is insufficient mechanical strength [25]. When collagen is used alone then rapid degradation is the main problem [26]. To modify the in-vivo degradation rate and to optimize mechanical strength, generally, natural polymers are mixed with collagen [27]. Collagen is an incredibly interesting biopolymer that can be blended with other synthetic or natural polymers such as chitosan, elastin, keratin, and silk fibroin for the preparation of new materials [21]. Different types of collagen materials have been used in biomedical applications with the integration of other biopolymers. These materials include collagen hydrogels, microfiber collagen scaffolds, electrospun collagen nanofibers collagen nanocomposite, collagen membrane, and collagen-containing sponges [28], [29], [30], [31]. Besides, gelatin is another biopolymer converted from triple helix collagen by heat into single-stranded molecules using more than base one for less immunogenic and allows enhanced cell adhesion ability [32,33]. However, both of these have significant applications in tissue engineering and wound healing [33]. Recent inventions of collagen with different polymers are presented in Table 1 along with material fabrication techniques, applications, and characterization techniques. The table lists the names of the tests that will be performed to determine the feasibility of collagen-based materials for biomedical applications.
Table 1. Recent inventions of collagen with fabrication techniques and applications.
| Materials | Fabrication | Characterization techniques | Applications | Ref. |
|---|---|---|---|---|
|
Hydroxyapatite/ Collagen/Chitosan |
Phase separation and freeze-drying | FTIR, SEM, porosity, density, XRD, swelling, vitro cytotoxicity and cell culture, stability, vitro cell culture (rabbit), tensile strength, biodegradability, radiological analysis | Alternative treatment in bone defects and bone tissue engineering | [34] |
| Chitosan/Collagen | Freeze-drying | Hemostatic capability (rabbit), vivo bio adhesive strength, cell culture | Hemostatic dressings | [35] |
| Chitosan/collagen | Electrospinning and situ crosslinked | SEM, WVTR, TGA, FTIR, tensile strength, cell culture, vivo wound healing property, histological | Wound healing | [36] |
| Chitosan/Collagen/ alginate composite dressing | Paint coat and freeze-drying | SEM, swelling, porosity, degradation, tensile strength, histological, vivo wound healing property, cytotoxicity, hemolysis | Wound healing | [37] |
| Chitosan/collagen | Blend cross-linking | Mechanical properties, optical properties, FTIR, cell culture, water content | Implantable corneal tissue engineering | [38] |
| Chitosan/collagen/ gelatin | A multistep procedure involving freeze-drying | Cross-linking degree, cell culture, antioxidant activity, Biodegradation, swelling, WVTR, MTT, SEM | Wound healing | [39] |
| Chitosan/collagen | Solvent evaporation | FTIR, tensile strength, and elongation at break | Wound dressing | [19] |
| Freeze-drying | FTIR, DSC, SEM, wound healing property, 1H NMR | Wound healing | [40] | |
| Crosslinked casting | SEM, TGA, FTIR, swelling, degradation, cell culture | Cornea and skin tissue regeneration | [41] | |
| Lyophylization and phase separation | SEM, porosity, hydrophilicity, tensile test, vivo study | Nerve tissue regeneration | [42] | |
| Chitosan/collagen/ hydroxyapatite | Phase Separation and freeze-drying | TEM, SEM, FTIR, XRD, DSC, tensile strength, amino acid count | Artificial bone matrix | [43] |
| Chitosan/collagen/ tannic acid | Freeze-drying | FTIR, tensile strength, SEM, Swelling, | Potential biomedical field | [44] |
| Chitosan/collagen/ ginseng compound | Freeze-drying | SEM, FTIR, XRD, swelling, density, porosity | Bone regeneration | [45] |
| Chitosan/collagen/ PLLA-CL | Electrospinning | Tensile strength, SEM, degradation | Tissue engineering | [46] |
|
Polyurethane/ collagen |
Coaxial electrospinning | SEM, TEM, AFM, XPS, FTIR | [47] | |
| Elastin/fibrin/ collagen | 3D fabrication | SEM, swelling, wettability and chemical cross-linking ability | Soft tissue engineering | [48] |
| Graphene oxide/ polycaprolactone/ chitosan/collagen | Electrospinning | SEM, FTIR, swelling, cell viability, cell degradation | Bone tissue engineering | [49] |
| Polypyrrole/chitosan/ collagen | SEM, FTIR, MTT, tensile test | Skin, nerve, heart muscle tissue engineering | [50] | |
| Chondroitin sulfate/collagen/ hyaluronic acid/PVA | Tensile test, cytotoxicity, FTIR, DSC, TGA | Tissue engineering and drug delivery systems | [51] |
Here, P (LLA- CL)-Poly(L-lactid-co-ε-caprolactone), SEM- Scanning electron microscopy, TEM- Transmission electron microscopy, AFM- Atomic force microscopy, XPS- X-ray photoelectron spectroscopy, FTIR- Fourier transform infrared spectrometry, XRD- X-ray diffraction, NMR- Nuclear magnetic resonance, DSC- Differential scanning calorimetry, TGA- Thermogravimetric analysis, MTT- Colorimetric assay for measuring cell metabolic activity, WVTR- Water vapor transmission rate.
The most recent in vivo research on collagen-based biomaterials is summarized in Table 2. For successful applications, collagen can be incorporated in various ways into porous scaffold, hydrogel, sponge, nanofibers, nanocomposite, biomaterials, bio-nanocomposite, film, and membrane. The dermatological animal takes 14th -25th days to form fibroblasts ECM for skin tissue regeneration and proliferation, according to the in vivo study.
Table 2. Recent vivo studies of collagen embedded biomaterials for biomedical applications.
| Materials | Fabrication techniques | Respective ratio | Applications | Findings summary | Ref |
|---|---|---|---|---|---|
| Chitosan/collagen | Freeze- drying | 3.0% chitosan and 1.0% collagen (7:3 w/w) | Wound dressing | Remarkable healing on 14thday at rat model | [52] |
| Lyophilization | 0.5 % chitosan and 1% collagen (3:1, 1:1 and 1:3 w/w) | Second degree burn wound | Chitosan/collagen (1:3) shows better efficacy on 25th day at rat model | [53] | |
| Chitosan/collagen/PEO/ curcumin | Electrospinning | 2.5% polymers concentration but different weight ratio of curcumin | Wound dressing | 15% curcumin shows 97% would closure on 20thday at rat model | [54] |
| Chitosan/collagen/AgNPs | Freeze- drying | 1:1 chitosan/collagen mass ratio with 0.15, 0.3, 0.6 or 1.2 mg/cm2AgNPs | Wound healing | Accelerate the 90% healing process of deep second-degree burn wounds on 20th day at rat model | [55] |
| Poly (ε-caprolactone)/ AIGIDs /fish collagen | 3D printing | 0.5% collagen was used in suitable poly (ε-caprolactone)/ AIGIDs | Bone tissue engineering | Strong osteoinduction capability in the rabbit tibia defect model for tissue implants | [56] |
| MAP/collagen | Film casting | 4 mg of collagen in 1 mL acetic acid with different MAP | Skin tissue engineering | Complete wound closure on 16th at rat model | [57] |
| Hydroxyapatite/collagen | 3D printing | * | Bone tissue engineering | Significantly increased new bone formation and ingrowth of blood vessels in vivo in a mouse model | |
| Elastin/fibrin/collagen | 3D crosslinked by 3% glutaraldehyde | 10% elastin, 2% fibrin and 2.26mg/ml collagen hydrogel grafted by stacking | Soft tissue engineering | The in vivo rodent model suggested new avenues for designing 3D grafts for soft tissue repair | [48] |
| Silica/collagen | Solution casting | 5 mg silica particles and 1 ml of collagen colloid (5 mg/ml) | Bone tissue engineering | In a Wistar rat model, silica particles stimulated new bone formation | [58] |
| Chitosan/collagen/PEO | Electrospinning | Chitosan and collagen mass ratio 1/3, 1/2, 1/1, 2/1, and 3/1 in 5% PEO | Wound dressing for skin regeneration | No cytotoxicity toward growth of 3T3 fibroblast | [59] |
| Chitosan, Chitosan/Collage, and Chitosan/Gelatin | Situ preparation | 5% (w/v) chitosan and 3% (w/v) collagen at different ratio | Wound dressing | Chitosan/collagen and chitosan/gelatin provides lower mechanical strength for stretch- shaped cells, stiffness of chitosan/gelatin close to 10 KPa, chitosan/collagen shows reduced cell attachment | [60] |
Here, PEO-Polyethylene oxide, AgNPs- Silver nanoparticles, AIGIDs-Abalone intestine gastro-intestinal digests, MAP- Magnesium ascorbyl phosphate.
*- Data not available.
The endeavor has been taken to focus on the recent development of collagenous material properties (e.g., bioactive, mechanical, viscoelastic, tensile, etc.) along with the source, structure, and extraction for the use of biomedical applications. The abundance of collagen protein sourced from nature may have potential applications in human tissue scaffold, cardiac implantation, wound healing, cornea membrane, dental membrane, dermal filler, cosmetic surgery, etc. as highlighted in this review.
Structure and types of collagens
Triple helix rod-like 29 collagen (Table 3 briefly discusses types and functions) composing of three α-chain characteristic variants with 1.4 nm (14 Å in width) molecules have been recorded where each about 280-300 nm (2800-3000 Å long) marked as a network forming polypeptide protein variants as I, II, III, IV, and so on [7,61,62] While two amino acids are identical and remaining has a different composition. Amongst the variants, widely used type I, type II, type III, type IV and type IV, which could be essentially practiced in bone cartilage, ligaments, dermis, dental, skin vessel wall, and lung treatments for a long time [63]. Type I collagen protein is the extracellular matrix that reduces fine lines and wrinkles and improves skin elasticity, also muscle support, and strengthens the nails, and hair of the various connective tissues with over 90% of the whole human body protein content. Type I and III respectively improve cartilage foundation and joint of connecting tissue, and supports gut health and eye health. Each α-chain of 18 amino acid residues per turn generally developed in the form of an extended left-hand helix structure [64]. Triple helix collagen is genetically composed of 3 polypeptide alpha chains having two identical chains (α1) and slightly different chains (α2) each with at least 1000 amino acid residues [61,65]. Besides, this one is sufficiently used in laboratory cell culture studies. Structurally, the proposed three chains together form a 3-phases wound healing platform by generating a hydrogen bond among the side chain of glycine to -NH peptide of hydroxyproline and =CH of proline. Since glycine, proline, and hydroxyproline/hydroxylysine are abundant in collagen, so the structure of collagen is largely centered on intra- and inter-chain hydrogen bonding, with GLY-X-Y standing for as previously depicted in Fig. 2. Approximately 4-8 collagen molecules are attached with covalent bonds forming collagen fibrils [66], where X indicates proline and also Y indicates hydroxyproline. Sometimes modifications of a certain amount of lysine and proline residues are hydroxylated through enzyme dominating bio-synthesis. The intermolecular weak bonds like hydrogen for triple helix structure stability depend on the content of 4-hydroxyproline [64]. Additionally, the basic formula of collagen is C2H5NOC5H9NOC5H10NO2. The physical structure of collagen fiber is shown in Fig. 1 and the chemical structure of the main components of collagen is shown in Fig. 2.
Table 3. Classification of protein collagen with details [[67], [68], [69]].
| Collagen type | Location | Synthesizing cell | Functions | Molecular composition | |
|---|---|---|---|---|---|
| Fibrillar | I | Skin, bones, tendons, cornea | Fibroblasts, osteoblasts | Resists tension | [α 1(I)]2α 2(I) |
| II | Gristle, vitreous body | Chondroblasts | Resists pressure | [α 1(II)]3 | |
| III | Skin, vessels, intestine, uterus | Fibroblasts, reticular cells, smooth muscle cells | Resists pressure Forms structural framework | [α 1(III)]3 | |
| V | Skin, bones, cornea, placenta | Endothelial cells associates | Associated with type I collagen | α 1(V), α2(V), α3(V) | |
| XI | Gristle, intervertebral disc | Epithelial cells | Provides tensile strength | α 1(XI) α 2(XI) α 3(XI) | |
| XXIV | Bones, cornea | Endothelial cells | Unknown | ||
| XXVII | Gristle | Unknown | Unknown | ||
| Non-fibrillar | IV | Basal membrane, capillaries | Fibroblasts mesenchyme cells, | Basement membrane, Forms meshwork to provide support and filtration | [α 1(IV)]2 α 2(IV); α 1 – α6 |
| VI | Bones, vessels, skin, cornea, gristle | Fibroblasts, mesenchyme cells | Microfibrillar, bridging between cells and matrix | α 1(VI), α 2(VI) α 3(VI) | |
| VII | Mucous membranes, skin, bladder, umbilical cord, amniotic fluid | Epidermal cells | Forms anchoring fibrils that fasten lamina | [α 1(VII)]3 | |
| VIII | Skin, brain, heart, kidneys, vessels, bones, gristle | Epidermal cells | Hexagonal network-forming, tissue support. porous meshwork, provide compressive strength | [α 1(VIII)]2 α 2(VIII) | |
| IX | Cornea, vitreous body, gristle | Epithelial cells | FACIT collagens, associates with type II collagen | [α 3(X)]3 | |
| X | Gristle | Epithelial cells | Hexagonal network-forming, Acts as calcium binding | [α 3(X)]3 | |
| XII | Gristle, tendons, skin | Fibroblasts | FACIT collagens, associated with type I collagen | [α 3(XII)]3 | |
| XIII | Skeletal muscles, heart, eye, skin, endothelial cells | Fibroblasts | Transmembrane collagen, cell-matrix and cell, adhesion | [α 3(XIII)]3 | |
| XIV | Vessels, eye, nerves, tendons, bones, skin, gristle | Fibroblasts | FACIT collagens, modulates fibril interactions | [α 3(XIV)]3 | |
| XV | Capillary vessels, Ovaries, heart, testicles, skin, placenta, kidneys | Endothelial cells associates | Multiplexing, proteolytic release of antiangiogenic | [α 3(XV)]3 | |
| XVI | Heart, skin, kidneys, smooth muscle |
Endothelial cells associates |
Multiplexing | [α 3(XVI)]3 | |
| XVII | Skin | Epithelial cells endothelial | Transmembrane collagen, cell to matrix attachment | [α 3(XVII)]3 | |
| XVIII | Kidneys, lungs, liver | Endothelial cells | Multiplexing, proteolytic release of antiangiogenic facto | [α 3(XVIII)]3 | |
| XIX | Skin, kidneys, liver, placenta, spleen, prostate gland | Unknown | FACIT collagens | [α 3(XIX)]3 | |
| XX | Corneal epithelium | Unknown | FACIT collagens | [α 3(XX)]3 | |
| XXI | Stomach, kidneys, vessels, heart, placenta, skeletal muscles | Unknown | FACIT collagens | [α 3(XXI)]3 | |
| XXII | Tissue connections | Unknown | Unknown | [α 3(XXII)]3 | |
| XXIII | Metastatic carcinogenic cells | Unknown | Unknown | [α 3(XXIII)]3 | |
| XXV | Eye, brain, heart, testicles | Unknown | Unknown | [α 3(XXV)]3 | |
| XXVI | Testicles, ovaries | Unknown | Unknown | [α 3(XXVI)]3 | |
| XXVIII | Nervous system Cells | Unknown | Unknown | [α 3(XXVIII)]3 | |
| XXIX | Skin | Unknown | Unknown | [α 3(XXIX)]3 | |
Here, FACIT- (Fibril associated collagens with interrupted triple helices).
 Fig. 1. Structure of collagen fiber [63].
Fig. 1. Structure of collagen fiber [63]. Fig. 2. Chemical structures of the collagen main constituents [70].
Fig. 2. Chemical structures of the collagen main constituents [70].On the contrary, collagen can be converted into gelatin which is very much soluble in water and makes viscous liquor by splitting some amino acids through boiling temperature. But high temperature can damage the triple helix structure and protein integrity as well. Under these circumstances, proteolytic enzymes or trypsin are suggested for digestion with minimum temperature [71]. Nowadays, collagen polymer with the natural antiseptic herb is being effectively fabricated for the intended use in wound dressing or tissue engineering. In recent years, the medical sector is getting increasingly interested in wound dressing materials that contain medicinal herbs instead of metal nanoparticles to impart antibacterial or other desirable properties. The practice of using medicinal plants to treat human skin including the healing of wounds and burn injuries, antifungal, antiviral, and antibacterial applications against skin infections has a long history and is going on for primary healthcare even in the modern age [10,[72], [73], [74], [75]]. Usually, wound dressing materials are broadly used for the treatment of skin wounds which are prepared from the textile fabric incorporating metal nanoparticles to impart antibacterial or other desirable properties.
Source of collagen
Collagen (glue-producing constraints), a natural polymer found especially in fibril-forming proteins in skin, bone, tendon, and cartilage can be applied diversely in food, cosmetic, and photographic pharmaceuticals industries [76]. Acid, acid enzyme, neutral saline solution individually or combined ultrasound extractions method are available, its application is determined depending on the field to be used. The high worth of commercial collagen has explored additional extraction from different natural species. Although mammalian species contain collagenous protein, non-mammalian species are offered importance due to human body resemblance and large economical sources. Collagen is sourced from fish, birds, bovine, marine, kangaroo tail, chicken feet, horse tendon, frog bone and skin, rat-tail tendon, sheepskin, and sometimes even from humans [13], but pigs' skin banned for muslin country [76], [77], [78], [79]. Starting back in the 1930s, porcine skins and bovine are the most commercial source of collagen. But fish scales have turned of great importance for religious sentiment to lessen the danger to acquire unknown pathogens [64]. Therefore, a lot of research has been done to find an alternative source of collagen. Since every country produces a large number of waste scales, the industry's attention is to alternative sources of mammalian collagen. On the other hand, type I collagen from fish scales is similar characteristics to mammalian collagen alongside marinated or salted skins that have very low amino acid (protein) content as compared to cold-water fishes [64,80].
Properties of Collagen
Collagen differs from globular protein enzymes for its long fibrous structure. It is absorbed with the catabolic action motivated by collagenolytic enzymes and phagocytosis [8]. Collagen is an outside part of the ECM that is found in crystalline form and supports the cells or tissue. It has high tensile strength and is composed primarily of ligaments, muscle, bone, skin, lens, and cornea of an eye [81], [82], [83]. Study reveals that collagen fibrils in older skin are fragmented and unevenly distributed, in contrast to those in younger skin, which are plentiful, compact, and well-organized [81]. But it offers tissue strength (a small amount), elasticity, and growth of new tissue as well [1]. Teruo Miyata et. al. prescribed collagen properties in their work as biodegradable, biocompatible, easily available, highly versatile, cell compatibility, possessing water affinity, high tensile strength, body absorbability, etc. [8]. The bioactive properties refer to the gum-forming or gel-forming ability of collagen [84], which helps to heal our cells. The major stages of wound healing are hemostasis, inflammation, proliferation, and tissue remodeling, with the last two being particularly concerned with collagen when scar tissue begins to form over the wound. Collagen is essential during the tissue remodeling phase for controlling tissue architecture and restoring strength to damaged skin. Skin scarring as a result of burns, surgery, and injury places a significant burden on the healthcare system. Patients with significant scars, especially children, have long-term functional and psychological problems. Scar tissue is mostly made up of type I and type III collagen [85]. Collagen type I synthesis is largely regulated by cytokine transforming growth factor β (TGF-β); a key driver of scar formation and fibrosis [85,86]. This is formed during the remodeling stage when collagen rearranges and restructures itself to resemble the skin's original structure. Scar tissue, however, differs from normal tissue in that it cross-links in a single direction rather than forming a basket weave. As a result, the functional quality of this collagen scar tissue alignment is usually inferior to that of the normal collagen randomized alignment. However, some tissues (for example, bone) can heal without causing structural or functional damage.
Collagen shows superior biocompatibility properties and a lower cytotoxic effect. The overall bioactive properties provide vital applications in biomaterials science [71]. A bioactive process on collagen in the human body related is always flexible and hydroxyapatite (HA solely used for hard tissue regeneration), is extensively practiced in bioactive coated platform to ameliorate the synergistic fusion between implantable scaffold-like bone, due to having similar natural apatite in bone. Some fish skin is a very natural treatment proper bioactive system and appropriates like tilapia fish body skin help to recover human body skin treatment.
It should be noted that skin contains eight different types of collagen, each of which contributes to smooth, firm, and robust skin. Enzymatic hydrolysis of triple helix building leads to the formation of oligopeptides. Furthermore, when collagen builds up on the skin right after being converted into bioactive di- and tri-peptides, it is released into the bloodstream and creates a bio-matrix [[87], [88], [89]]. Collagen get helps to heal the damaged tissue of our skin quickly by its supreme properties. Extensive studies were carried out on nanoscalematerials including elastic properties but fewer investigations were performed at the microscopic scale especially on albuminoid molecules and fibrils. These scales are important for an entire understanding of the role of collagen as a vital component within the extracellular matrix. Researchers perform silico creep tests using an atomistic modeling approach to an albuminoid-like amide to watch the creep for various external applied loads. The result shows that individual albuminoidal molecules show a nonlinear elastic behavior with young's molecule increasing from six to sixteen standard consistence of 3.84 (±0.38 Pa) and time constant within the vary of 0.24-0.64 ns. It is recommended that the viscose behavior of collagen fibrils and fibers involves additional mechanisms, adore molecular slippery between scleroprotein molecules inside the fiber, or the result of relaxation of larger volumes of solvent [90]. M.C. Gomez-Guillen added that audited antioxidative and medication properties have presumptively been combined with this single organic compound composition [66]. The biological activities of the super molecule hydrolysatesare concerning the amino acid composition, sequence, size, and configuration of peptides [91].
At present, while determining the mechanical properties, collagen fibers with a diameter of about 50 to 200 nm provide some help in the protein-like structure. However, upon only the concept of scientists, the mechanical properties of collagen are still inconclusive, but limited mechanical properties are the main shortcomings [92]. Because of its role, its excellent mechanical properties are very important. So crosslinking fibrils network with previously mentioned improve the mechanical properties at elevated temperature with appropriate abrasion [93]. Collagen nano fibrous mats are nowadays being used in various organ tissue improvement scaffolds with superior mechanical properties and flexibility [94]. Biomaterials template offers temporarily mechanical support with additional cell adhesion and proliferation facility to the damaged organ in human skin [95]. Collagen-based nanomaterial's strength depends on several factors (material formation techniques, porosity, thickness, nanofiber diameter, degree of crystalline and amorphous regions, some extraneous factors, etc.) as studied by L. young et al. [95]. Moreover, synthetic chemicals, besides natural polymer are practiced to improve mechanical strength to a great extent and enhance low wear strength [96]. It is believed that the tissue's ability to approach high levels of stress is due to the proportion of large-diameter fibrils in the tissue. Changes in tissue mechanical behavior can also be attributed to changes in the content and composition of non-collagenase components in the tissue including proteoglycans, glycosaminoglycans, and elastic fibers [97]. A study showed that incubating corticosteroids HC (high concentration) or LC (low concentration) for 3 and 7 days adversely affects the mechanical properties of the separated collagen bundles, without observing cross-linking. The ability of the tissue to approach high-stress levels is said to be the share of huge diameter fibrils within the tissue. Variations in the mechanical behavior of tissues also can be attributed to variation in content and composition of no collagenase parts gift within the ECM, including proteoglycans, glycosaminoglycan, and elastic fibers [98].
Due to the lack of sustainable and biocompatible substitutes for tendons and ligaments, the situation of tissue replacement is very important in the pharmaceutical industry. The tendon made of tissue should completely reduce the transplantation failure of autologous source care cells. In addition, the construct can be adapted to its physical conditions through preliminary implantation. According to the scaffolding data, the tangential module is closer to the module found in the body of an adult tendon [99]. Now it has been overlooked that collagen affects cell integration (chemotactic, adhesion, migration, and morphology), etc. Also, polymeric collagen-platelets aggregations lead to hemostasis effects of human body cells.
By the structure of native crosslinks, collagen in biological tissue is strong and provides enzymatic fibrillary degradation rapidly when scaffold come in contact with human tissue. To mitigate the problem, various cross-linkers (physical, chemical, natural) are needed to induce intra and intermolecular which sufficiently stagnate the existing collagen in developed biomaterials for biological tissues. Moreover, crosslinks additionally may be fashioned among adjoining microfibrils if the distance among microfibrils is smaller than the period of the crosslinking agent introduced [100]. Grant et al. studied the characteristics of collagen fibrils through nanoindentation to investigate the radial compressive mechanical properties in the course of several saline solutions with various compositions, concentrations, and pH. It is located that growing the salt intentness brought about a numerous boom in modulus. This is in settlement with current modeling consequences displaying that the number one impact of cross-linking is at the failure mechanics instead of the modulus. It is feasible that acting the test on reconstituted fibrils might result in extra salt sensitivity than what is found in the study [101][102]. This is a particular surface area to quantity the problem. As the crosslinked fibril number increases with the generation, the surface area improves less quickly. Accordingly, the surface exchange with the glycosaminoglycan matrix comes to be very reduced when compared with large fibrils. The current analysis conjointly suggests that different characteristics might play a foothold within the high-speed strength of the membrane, the level of intrafibrillar crosslinking, and the bulk vs bound water volume of the tissue sensitivity against tensile strength to differences in test rate existed greatest in the youngest tissues, which have the lowest quantity of crosslinked collagen both intrafibrillar [103]. Due to the extremely anisotropic structure of sclerostin fibrils, the mechanical response in the radial direction is significantly different from the mechanical response in the axial direction [101].
Extraction protocol of fish collagen
This section is going to discuss the extraction protocol of fish collagen as presented below. Wastage carp fish scales are assembled from the market and stored in a fridge after properly washing with fresh or double-distilled water for 15 minutes.
Demineralization
The scales will be immersed in alkali solutions (eight times to fish scales) 0.1 M NaOH [104,105] for the whole day to remove non-collagenous fats and impurities by complete striation with altering the alkali solution every 2 h respectively. Until getting a neutral pH, it will be washed thoroughly with double distilled water. After that, demineralization takes place in an acid solution soaking (50 ml HCl 0.2 M, 50 ml H2SO4 0.5 M, and 80 ml H3PO4 0.5 M) for only a moderate time (20 minutes at twenty times acid solution to fish scales)[104]. In addition, after changing the solution every 12 hours, the fish scales are soaked in 0.5 M ethylenediaminetetraacetic acid (EDTA) with pH 7.4 for 24 hours, then continuously stirred (9000 rpm), and then soaked in a neutral pH distilled water 3 times to perform double desalination [[106], [107], [108], [109], [110], [111]].
Extracting collagen (acid soluble collagen)
The scales after demineralization are immersed in an acetic acid solution (CH3COOH) of 0.5 M [112]. Then it should be kept under 24 hours of magnetic agitation to extract the collagen protein. Importantly, the solution needs to be mixed with a shaker at a high speed of approximately 20000 rpm for 30 minutes to improve the contact between the flakes and the acid solution. Then, the solution is filtered through Whatman paper 1 for obtaining the collagen solution. After the filtration, a brine solution of NaCl 10% is added to the previous filtered solution to get raw collagen as it is precipitated (supernatants are salted out) from the solution. An osmotic membrane process will be performed for 48 hours until re-precipitation in an acetic acid solution (CH3COOH 0.5 M) is observed and then purification will be carried out with distilled water. Finally, the purified collagen flakes must be freeze-dried and stored in a refrigerator. The total extraction process would be executed at 4°C [[113], [114], [115], [116], [117], [118]].
Biomedical applications of collagen
Triple helix elongated fibrils collagen macromolecule from amino acids constituent bonds collectively known as a collagen helix. Collagen has many applications in treating difficult bone and skin problems. A huge amount of collagen can be found in different body channels like corneas, gut, blood vessels, intervertebral dice, etc. Collagen is one of the most promising natural proteins used in the early stages of tendon production in various animals, and is beneficial to the human body. To date, there are lots of commercial products are available in market for the treatment of damaged body organs as collagen peptides powder, collagen gel, collagen ointment, collagen face mask, collagen lotion, face pack, NeuraGen®, NeuroGen®, NeuroFlex®, NeuroMatrix® etc. The most important recent biomedical applications of collagen are reviewed and presented in Table 4.
Table 4. Application of collagen bio-materials in medicine [68,119,120].
| Biomaterial type | Medicine areas | Applications | Drug delivered |
|---|---|---|---|
| Fibroblasts hydrogels | Skin regeneration | Reproduction of skin defects | - |
| Hydrogel with liposomes | Skin regeneration | Vitro and in vivo skin reconstruction | |
| Sponges | General surgery | Hemostasis | |
| Dentistry | Oral wounds | ||
| Dermatology | Soft tissue augmentation | - | |
| Orthopedics | Bone repair | - | |
| Urology | Bulking agent for incontinence | - | |
| Cardiovascular | Arterial puncture repair | - | |
| Infected lesions | Drug delivery | Gentamycin, cefotaxim, fusidic acid, clindamycin, all-trans-retinoic acid | |
| Membranes | Nano-composite | Wound dressing | Propranolol hydrochloride |
| Dentistry | Guided tissue regeneration (GTR) | - | |
| Film, sheet, and disc | Cancer therapeutics | Drug delivery | Medroxyprogesterone acetate, human growth hormones, tetracyline |
| Microsphere, nanosphere | bone tissue engineering | Drug delivery | Retinol, tetracain, tretinoin, lidocain |
| Damaged organ or tissue | Carrier for cell culture | - | |
| Shield | Ophthalmology | Corneal | Gentamicin, vancomycin, tobramycin, trimethoprim |
Cardiac application
The amount of blood pumped as heart output to the left and right ventricle in one minute is normally expressed as cardiac physiology [121]. Transparently, the amount of blood pumped known as stroke volume (SV) is calculated as the total amount of blood divided by total heartbeats per minute (bpm). Heart collagen is composed of four heart valve rings. These rings are histologically flexible and have unique safety for completely different internal muscles. Collagen can promote the vitality of internal organs, which is the relationship between the torsion force per unit area removed from the heart and the mechanical force. Its structure separates the upper chamber from the center of the lower chamber because as long as the body cavity is fibrillated, the support for albumin fibrillation will not decrease. The massive quantity of macromolecules in the viscus is fibrillar collagen. It is currently typically accepted that aging is related to albuminoids collected from several organs including the heart. Studies have shown that collagen levels in the main (left) ventricle increase with age and are related to wall tension and dysfunction. Cardinals of similar age can be found in various animals [122,123]. Another study reveals that age is the independently main predictor of extracellular volume fraction [124]. So, nowadays collagen can be used for different aged people for preventing difficult diseases like heart attacks, diabetes, etc. The main function of sclerostin in the heart is to create a structural basis for the cardiomyocytes of internal organs, provide flexibility for the myocardial wall, and promote force transmission. However, the regulation of the collagen matrixonly plays a regenerative effect in the case of scars after trauma, which stops the stimulation of the wall [125,126]. Nowadays, people often find that with age, collagen will be stored in various organs and the heart. Similar results were obtained in the rat model, and the results showed that from the age of 55, the amount of lovely albumin will increase with age. And related to wall tension and malfunction [127]. This suggests that fifty-five pathologies may cause changes in pulsation or diastolic blood pressure, but this is not the case, indicating that age-related pathologies are not necessarily related to the outcome of hypertension care. This pathology has even been found in giant animal models (including sheep and dogs) [122]. Recent clinical studies are using imaging technology to reduce the area of open fibrosis in the body, the size of scars, and the relative volume in the body, as an alternative to biopsy from body cavities [128]. Studies over 1200 patients aged 54-93 years using internal organ resonance images show late gadolinium enhancement. Collagen, elastin, laminin, glycoproteins, and glycosaminoglycans are all components of cardiac extracellular matrix [129]. Sternal wound infections (SWI) are a major concern following cardiac surgery because they result in a longer hospital stay, increased morbidity, mortality, and hospital costs. Gentamycin Containing Collagen Implants (GCCI) are successfully and effectively used in cardiac surgery to reduce sternal wound complications in over 50 countries. Furthermore, GCCI provides high local concentrations of gentamicin while maintaining low serum levels. It is worth noting that Collatamp is rapidly absorbed by the wrapped sternal edges and does not require surgical removal [130]. Compared to control group, GCCI shows better results always in terms of surgical revision and antibiotic usage. As a scalable approach for diagnosis, therapeutic monitoring, and prognosis, collagen-based bio-makers reduce myocardial fibrosis (cardiovascular diseases) [131,132].
Wound healing application
Wound healing refers to replacing broken or torn tissue with newly created tissue. In this human article, physical fitness replaces wound healing and leads to the post-traumatic recovery process. In healthy skin, the epidermis and dermis can protect the skin and act as a barrier to the external environment. Once the barrier is broken, a controlled sequence of organic chemical events is triggered to repair the damage [133,134]. Human skin wound healing consists of four dynamic overlapping stages hemostatic, inflammation, proliferation, and remodeling [135]. The wound is difficult and fragile to heal, it is believed that once interrupted or failed, leading to the formation of chronic non-healing wounds such as diabetes, vascular disease, infection, and metabolic disease [136]. Wound care accelerates wound healing by cleaning and preventing repeated injuries or infections. Albumin is resistant to bacteria that are commonly found in wound dressings. Healthy granulation tissue will therefore form and heal the burn quickly as a result of adding antibacterial collagen to the burn dressing [137]. So, it can be said collagen serves fibroblasts and migrates beside connective tissue. The massive area of scleroprotein fibers will attract fibro genic cells that facilitate healing. Blood platelets of our physical structure interact with scleroprotein to form an astringent plug. A scleroprotein act as ‘glue’ that serves to plug that breaks within the vas preventing more bleeding [138,139]. Natural dressings that contain protein have certain properties, while artificial dressings do not. Because it can resist bacterial infections, it helps to keep wounds ineffective. In the four stages of wound healing, collagen performs the following functions. It has the following guiding functions, this fibrous polymer is used to guide fibroblasts: they migrate together with the animal tissue matrix, chemotactic properties (a large number of collagen fibers attract fibroblasts that promote healing), embryos (there are binding the neutral salt molecule acts as a nucleating agent that induces the formation of the fibrous structure. Proteinase wound dressing can act as a conductor to balance the deposition of new tissue and hair growth.
Tissue engineering
The design of tissue engineering (promising alternative treatment of osteochondral damage [25]) biomaterials for a contractile organ application needs a replacement style chest got to balance needs biomechanical and bioactivity [95]. Tissue engineering refers to the medical specialty engineering discipline that uses a galaxy of cells, engineering, materials process, and correct organic chemistry including restoring, maintaining, improving, or replacing physicochemical factors completely of different types of biological tissues [140]. Its objective is to replacement of broken or lost tissue for building new living tissues, expectant to maintain and enhancement of half or the whole organ of living organisms [141,50]. Nuge, T et. al added chemically treated synthetic polymer-based scaffold in the biomedical field to unfold the applications for offering sufficient tension, moisture management property, porosity, demanding degradation rates, etc. [142]. But the synthetic platform has some detrimental effects on humans direct or indirect. Collagen macromolecules moderate to high hydrophilic nature to mimic the tissue requirements. So far, examples of tissue damage are nothing but bone is considered as tissue. If one that bone got fractured, then obviously consult the hospital and the doctor will do tissue engineering technique on the particular damaged tissue in such a way that bone which has been fractured will be recovered. Here recovered bone from a fracture from the damaged position is named tissue engineering [50].
Collagen-based 3D biomaterials for cell culture and tissue engineering have received a lot of attention in recent years as an additive technology. Layer by layer cell-laden hydrogels, known as bio-inks, can be assembled to create personalized need-based features. Bio-inks are soft biomaterials that contain living cells. But selection of proper bio-inks is the major drawbacks. Print speed influences the printability of collagen bio-inks, but as previously stated, mechanical properties are the major drawbacks [143]. To address the issue by improving manufacturing process, a collagen injectable bio-ink enabled process (5-10 mg/ml collagen) is carried out inside the secondary hydrogel (e.g., gelatin slurry). There have been a few studies on collagen bio-inks that do not contain any additives. Another way to improve printability is to maintain the proper storage modulus (depends on collagen concentration, pH, temperature, NaCl amount in bio-inks) before extrusion. Collagen has a significant influence on the geometric fidelity, cell viability, and mechanical properties of printed constructs. One of the most important factors is collagen concentration, which is normally 80 mg/ml (also known as Viscoll Bioink) for increasing storage modulus [144]. But collagen concentration does have any influence on cell viability. Highest printable concentration offers elastic modulus to 30 KPa. 3D bioprinting of collagen ink into a cell-laden biomaterial has no cytotoxic effect and cell viability of over 70% when tested on Vero and NIH 3T3 cells [143]. Hydrolyzed collagen (gelatin) is preferred for bio-inks because it has better physical properties than pure collagen while maintaining cell adhesion and biocompatibility. Different natural derived hydrogels for bio-printing have been well studied by H. Li et al., and supported collagen and gelatin show good printing ability compared to other hydrogel materials [145]. Extrusion bioprinting of cell-laden 3D scaffolds was used on a mouse model, and the results showed complete recovery of damaged skin after 1 week [146]. However, bilayer to several layer embedded 3D porous materials derived from pure collagen, fibrinogen/collagen, alginate/collagen, gelatin/collagen, chitosan/collagen, agarose/collagen, hyaluronic acid/collagen and bio-ceramics/collagen were applied to full-thickness wounds, with the results demonstrating rapid wound closure, reduced contraction, and accelerated re-epithelialization [144,147].
The three main components are mainly used in tissue engineering such as B. cells (such as stem cells, adult stem cells, and embryonic stem cells), scaffolds (such as metals, ceramics, polymers, and their compounds), and signaling molecules (such as growth, factors, hormones). Stem cells do not divide in culture and can produce different forms of specialized cells. Scaffolds in tissue engineering of intervertebral discs, the scaffold methods of tissue engineering are given in the tissues and appendix. In this review, it was selected as a representative example. Many efforts have been made to find biological treatments for different severity of intervertebral disc degeneration. With early disc degeneration, the disc cells, especially the nucleus pulposus cells, cannot synthesize the correct ECM. As the water content decreases, the gelatinous nano particles (NPs) become more fibrous. The main content of the extraction should be the ability of NPs cells to secrete the correct matrix components into special proteoglycans, which are responsible for the water absorption function of the nuclear matrix [141].
The bioactive molecules are biomolecules like growth factors hormones and genetic protein. All of these both scaffold as the signaling molecules will act as nutrient media for the cells. Once the cell becomes a scaffold and signal molecule, the cell undergoes cell proliferation and divides rapidly after proliferation. The victimization of composite materials is increasingly exploring the sum of synthetic materials and natural derivatives because they can achieve advanced properties related to the material system used. These compounds may include man-made polymers, natural biopolymers, or mixtures [148]. Scaffolding in intervertebral disk tissue engineering- deciding on the scaffolding method for tissue engineering is tissue and alertness specified. The intervertebral disk is selected as a consultant instance in this review. Mass efforts are created to head seeking out the organic medicinal drug for disc degeneration of numerous severities. In early disc degeneration, disc cells mainly the nucleus pulposus cells calm down successfully to synthesize the proper ECM. The jellylike NPs will become more fibrous with reduced water content. The primary to be repaired must be the capacity of the NPs cells to secrete the right matrix components in unique proteoglycans, which might be responsible for the water-soaking up the overall performance of the nuclear matrix [149].
Nerve tissue engineering
The nervous system is a critical component of the body, and its injury or damage can have serious or potentially fatal consequences. Because of the complex physiology and limited restoring capacity, treating damaged nerve tissue is a complex process. However, collagen polymer conduits for peripheral nerve regeneration have already been tested in clinical trials with success [150]. The nervous system serves as a command center, regulating the movements of each organ and the combination of physiological technology. Any type of nerve damage can reduce the quality of life. A vast network of nerves also sends and receives electrical signals throughout your body to and from other cells, glands, and muscles. The pressure on the nervous system promotes the application of tissue engineering strategies, that is, the damage of tissue engineering scaffolds promotes the regeneration of severed nerves [141]. Nerve regeneration may be a complex biological phenomenon in the peripheral nervous system. When the disease is small, the nerve will regenerate on its own, when the disease is large, surgery is required, usually a nerve transplant from other parts of the body [151]. Nerve guide conduits made of collagen have been used to repair minor nerve injuries (5 mm gap). They have, however, shown promising results in the regeneration of larger distances, such as a 15-mm gap in the rat sciatic nerveand partially reconstructing a 35-mm gap in the dog sciatic nerve model. Collagen is also used in conjunction with other polymers and proteins, and it is the only biopolymer that has received clinical approval for use in neural tissue engineering [152]. Collagen VI regulates Schwann cell differentiation and is required for peripheral nerve myelination, function, and structure, as well as nerve regeneration after injury. Although the role and distribution of collagen VI in the peripheral nervous system are well established, its role in the central nervous system, as well as its links to human neurological and neurodegenerative disorders, remain unknown [153]. Though collagen VI applications in the nervous system are becoming more prominent, more comprehensive knowledge of the central nervous system (CNS) and peripheral nervous system (PNS) is required [153,154]. The nervous system is assessed using the least stressful central nervous system (CNS) and peripheral nervous system (PNS) strategies. The central nervous system, which includes the brain and spinal cord, visual and sensory functions, and the auditory system, executes and interprets warnings and receives PNS stimulation. The PNS is made up of cranial nerves that branch off the brain, spinal nerves that branch off the spinal cord, and the body of nerve cells (the dorsal root ganglia) and their processes. Peripheral nerves transmit sensory and stimulating signals from the spine to innervate muscle tissue [155]. The physiology of the nervous system presents some challenges to the biotechnological research of neuralgia. A lyophilized collagen/chitosan composite has been successfully created for use in peripheral nerve regeneration. Chitosan provides mechanical strength and bacterial efficacy in this case, while collagen promotes cell attachment. This composite has the potential to be a promising candidate for bone, tendon, and muscle tissue engineering [42].
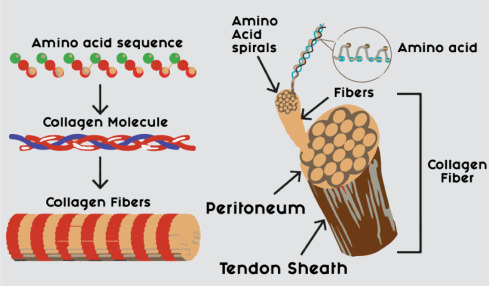
Tendon/ligament tissue
Tendons and ligaments are fibrous connective tissue, and collagen constitutes 70% to 80% of its net strength. Tendon structure is shown in Fig. 3 and each tendon and ligament has gentle natural activation ability, and never withstands full-thickness injuries. Tendon injury is a formal clinical crisis, and ligaments (mainly the ligaments around the sutures of the ankle and knee) are sometimes torn due to injury. Due to the recovery of the natural condition of damaged connective tissue or ligaments, there is currently no recoverable medical service [156]. Tendons are organized especially of water and sort one (85%) collagen, classified in graded levels of complexness. different forms of collagen are a plus at the enthesis, still, as around blood vessels [157]. Among all the reports of tendon revisions, there is no real technique that can completely restore the injury to the tendon and ligaments. The albumin framework provides an excellent method for repairing and strengthening tendons/ligaments [141]. The analysis disclosed that the collagen type I coating will function effectively as a useful technique for connective tissue injury repair. The electrospun texture will simulate the fibrous layout of the connective tissue and scaffolds do not support tendon/ligament tissue improvement [141]. This result predicts the actual possibility of using the largest diameter in the tissue engineering of tendons/ligaments. In further research, a large number of collagen gel packages with uniaxially oriented fibrils and larger sizes are being manufactured [141]. All of the higher-than studies prompt that an orientation system will improve the repairing potency of ligaments thanks to the property of resident tissues. Scaffolds that aspirant details and purposeful properties of tissue in vivo entirely offer a useful strategy for the renewal and repair of the connective tissue [141].
 Fig. 3. Collagenous tendon in cross-section [157].
Fig. 3. Collagenous tendon in cross-section [157].Tendon and ligament pathologies are usually seen by general practitioners, rheumatologists, and specialists in system medicine and sports and exercise medication, accrued participation in competitive exercise and sport. However, solely belatedly are these conditions receiving the eye they are from the research community [158].