Highlights
-
•
Microneedle patches can be used for drug delivery and disease monitoring.
-
•
They allow synchronous drug delivery and ISF biomarker monitoring.
-
•
Their use has been reported to result in significantly reduced pain.
-
•
Their use in tissue biopsy has recently been tested with promising results.
Abstract
Microneedle (MN) patches are composed of micron-sized needles organised in arrays and attached to the backing of a patch. The most common type is the transdermal patch, designed to uniformly penetrate the stratum corneum to reach the dermis of the skin. Recent advances in 3D printing technology have allowed the development of reproducible, efficient methods to create microneedles on a large scale, which had previously been a factor in the limited clinical uptake. In comparison to conventional drug delivery methods, MN patches have been shown to significantly reduce pain and scar generation while maintaining effective and reliable delivery of vaccines, immunotherapies, and slow-release drug therapies. The MN design has also been investigated as an alternative to conventional tissue biopsy, with positive results. Synchronous delivery of medications while monitoring biomarkers in dermal interstitial fluid (ISF) is also a promising clinical development with wide-reaching benefits. MNs are diverse in design and material composition, and with developments in fabrication technology, transdermal drug delivery has been applied to many clinical fields, including chronic illnesses such as arthritis or diabetes, cancer, immunotherapies, epidemic disease prevention and ocular treatments. While the majority of MN patch applications are still in the pre-clinical testing phase in animal models, further translation of this technology to the clinic could aid in medication and vaccine compliance, improve treatment access in rural and remote communities, improve targeted therapy applications and provide financial cost savings to the public health sector. This review evaluates the designs and applications of current transdermal MN patches for drug delivery, biomarker monitoring and diagnostic biopsies compared to conventional needle-based methods.
Graphical abstract
Keywords
Microneedle
Drug delivery
Vaccination
Diabetes management
Biopsy
1. Introduction
Pistor first described microneedle (MN) patches in 1975, and was awarded a patent for a device that could be used for cutaneous delivery of medications to provide pain relief and in the treatment of dermal disorders [1]. While there was a vision for the delivery of drugs through a MN patch, it was not until 1998 that successful transdermal delivery was demonstrated [2]. In this study, a Franz diffusion cell was used to demonstrate that transdermal delivery of calcein (a fluorescent dye) was possible in human skin specimens. A Franz diffusion cell has an upper and lower chamber, with the sample membrane separating the two chambers. Fluid is sampled from the lower chamber to allow identification of how much of the tested substance has permeated the membrane. The study showed that microneedles significantly increased the delivery of calcein compared to control specimens. Since these early studies, MNs have gone on to be used in a diverse range of clinical applications, such as drug delivery [3,4]and disease monitoring [5]. MNs have also been trialled as an alternative to conventional wide gauge needle biopsies for skin disease diagnoses [6,7], sampling interstitial fluid (ISF) biomarkers [8], and devices that synchronously sample ISF biomarkers for monitoring and facilitating drug delivery in response to biomarker levels [[7], [8], [9]]. The benefits of MN patches over conventional needle-based injections and biopsies include reduced pain, bleeding, bruising and vasovagal attacks [10], as well as overcoming needle anxiety and non-compliance [11].
This review will explore the designs and applications of current transdermal MN patches for drug delivery, biomarker monitoring and diagnostic biopsies compared to conventional needle-based methods. This will provide novel insight into the advantages of MN patches and their potential for increased applications in not only drug delivery, but also disease monitoring and tissue biopsy in the medicine field.
2. Design principles
MN patches are manufactured using a variety of materials and methods and consist of micron-sized needles attached to a base that can penetrate the skin with the depth of penetration adjusted to achieve the purpose of the MN patch [12] (Fig. 1). Microneedles used for drug delivery may utilise a design similar to that of Fig. 1a, with solid needles [13] loaded with a drug, or with fluidic channels, allowing the analysis of fluid removed from the sample or delivering a fluid [14].
 Fig. 1
Fig. 1Common materials that are used to fabricate MN patches include stainless steel, titanium, silicon, polystyrene, polymer hydrogel, polylactic acid (PLA), polyethylene glycol (PEG), sodium alginate and hydroxyapatite, polyvinylpyrrolidone (PVP), cycloolefin polymer, and poly lactic co glycolic acid (PLGA) [6,[15], [16], [17], [18], [19]]. Metals and silicon show good mechanical properties; however, the new generation of MN patches for drug delivery are made from dissolvable materials to avoid sharps waste and increase delivery efficiency. Additionally, some plastic polymers may have unfavourable interactions with the drug of interest or result in toxicity and be unsafe to use for extended periods [20]. These aspects must be considered when fabricating a MN patch to ensure it is fit for purpose.
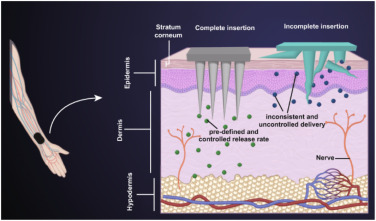
The optimal length of the needles is an important design principle to ensure sufficient dermis penetration [18] while reducing and minimising innervation to the nerve bed to avoid pain and blood vessels to avoid blood loss. Fig. 2illustrates the mechanism for reduced pain associated with MNs when used for both biopsy and monitoring, where the needles penetrate through the epidermis and into the dermis so that their payload can move into the blood vessels but do not contact nerves. Similarly, optimal spacing is an important design element, if the needles are too close together, they may not penetrate the dermis effectively, and too far apart, they may snap or bend, resulting in incomplete insertion and; therefore, impaired delivery of the drug (Fig. 2).
 Fig. 2
Fig. 2Manufacturing of microneedles meets many challenges, from design to manufacture. One of the major challenges faced by researchers is consistent reproducibility and designs which allow the delivery of drugs and/or monitoring to be feasible. In recent years, three-dimensional (3D) printing tools have been widely used to manufacture MN master devices and MNs for immediate use, and due to the use of computer-aided design (CAD) software and advancement in the field, reproducibility has been improved. The procedure to generate different types of MN master devices follows a standard process. First, computer-aided design (CAD) software is used to design the master MN patch. The design is then fabricated using a 3D printing device. Subsequently, a negative mould is created from the master mould. Finally, the negative moulds are used to create the final MN product using micro-moulding techniques, resulting in polymer or thermoplastic MNs [14]. Due to the recent advancements in 3D printing, such as increased reproducibility, increased reliability, and the ability to print thermostable vaccines which can be self-administered [21], this process is more accessible than in previous years.
3. Drug delivery applications
MN patches have great potential in the drug delivery space due to their versatile applications, with dissolving patches which can allow slow release of drugs or their pain free delivery. The clinical uptake of using microneedles for drug delivery has historically been delayed due to a lack of information surrounding the drugs which are able to be loaded into microneedles, and how to subsequently apply this to the relevant area. Recently, vaccines, diabetes management, ocular treatments, anti-cancer chemotherapy and antithrombotic medication have all been successfully tested (Table 1).
| Clinical application | Drug/Disease treated | Model | Benefits compared to conventional methods | Source |
|---|---|---|---|---|
| Vaccine |
|
|
|
|
| Diabetes Management |
|
|
|
|
| Ocular treatments |
|
|
|
|
| Anti-cancer chemotherapy |
|
|
|
|
| Antithrombotic medication |
|
|
|
|
3.1. Vaccines
Conventional methods of delivering injectable vaccines usually require attendance to a health clinic where the vaccine is administered by a healthcare professional. A major benefit of MN patch technology is that it can be administered and used by non-medically trained personnel. This is due to the ease of application and minimal pain response [19]. With the advent of clinical translation, this technology could assist in closing the gap in healthcare access, regardless of the place or country of residence. To date, research investigating the efficacy of vaccine delivery by MN patches has been undertaken using animal models.
Successful delivery of the SARS-CoV-2 vaccine was reported in a murine model (female BALB/C mice), resulting in immunogenicity [22]. In other applications, MN patches have been reported to effectively deliver the measles vaccine to non-human primates (Macaca mulatta) [23]. It was shown that delivery through a MN patch resulted in comparable immunogenicity to that of conventional vaccine delivery. MN patches have also been reported to be stable at higher temperatures for an increased period in both lyophilised and reconstituted states [30,31]. This is beneficial for the manufacturing process as it increases the time it takes for the product to remain stable without losing potency. Studies investigating the annual influenza vaccine have demonstrated stability at higher temperatures [30]. This suggests that MN patches have the potential to be removed from cold chain storage, which could reduce the costs associated with the storage and transport of conventional vaccines.
The financial benefit of MN patches to deliver the yearly influenza vaccine in the United States has been estimated during influenza season. Lee et al. [32] estimated a potential saving of US$950 million to the private sector and US$2.6 billion to the public healthcare system in a single season. This study considered many scenarios, including over-the-counter vaccines for individual administration and administration by healthcare professionals. Additionally, a price point of $9.50 to $30 per vaccine was used, with all price points resulting in a cost-effective result. This assumed equivalent or improved efficacy compared to conventional methods and a minimum 10 % market share [32]. These significantly reduced costs could improve the accessibility of healthcare to those in lower socioeconomic situations.
3.2. Diabetes management – medication delivery
MN patches have shown promise in the management of diabetes. Conventional management for type one diabetes mellitus involves regular blood glucose measurement using the pinprick technique, followed by the administration of insulin as required to maintain glucose homeostasis [33]. Type two diabetes mellitus is often treated with daily injections of liraglutide or lixisenatide [34,35].
Liraglutide is a glucagon-like peptide‐1 (GLP‐1) receptor agonist and has been shown to assist in managing glucose homeostasis in patients diagnosed with type two diabetes mellitus [35]. Liraglutide is a daily injection; as such, compliance can be poor, with less than 70 % of patients shown to be compliant over a study period of 40 weeks [36]. Unni et al. [37] also found poor adherence to prescribed medications in type two diabetes mellitus patients, with self-reported non-compliance being 27 % over three years. The factors attributed to medication nonadherence included inconvenience and/or forgetfulness. This non-compliance clearly shows the need for a more convenient, and likely less painful way to deliver the medication.
Rabiei et al. [19] developed a MN patch containing liraglutide. They demonstrated in vitro that there was a linear release of the drug for eight days, using 80 % of the total drug, through the use of a Franz diffusion cell application on biopises of hair-removed Wistar rat skin. They also calculated that these patches could be made with increased needles for use in humans and would be able to maintain clinically appropriate levels of liraglutide for seven days at initial (0.6 mg/day) and maintenance (1.2 mg/day or 1.8 mg/day) doses. This could be a significant relief to patients struggling to manage symptoms of type two diabetes, as they would only need to administer a new patch weekly instead of a daily injection. This supports other studies which have also investigated the delivery of liraglutide using MN patch technology [24,25]. You et al. designed a microneedle patch with a 3 layered design, utilising egg products to assist in the complete delivery of liraglutide. Common difficulties associated with the use of microneedles for drug delivery include the complete diffusion of the drug due to difficulties with the complete insertion of the needles and damage to the active drug during manufacture and storage. The egg-based microneedle patch negated these difficulties, with complete delivery of the drug reported.
Lixisenatide, a glucagon-like peptide-1 receptor agonist, is another drug used to help control the symptoms of type two diabetes mellitus [34,38]. MN patches containing microparticles of lixisenatide have been shown to help maintain glucose homeostasis in a type two diabetes murine model (db/db mice) [20]. The bi-layer MN patch was manufactured as a lixisenatide and PVP compound, with a dissolution time of only three minutes once applied. As the needles dissolve, the base can then be removed. The cytotoxicity of the PVP matrix on a murine fibroblast cell line (L929) and a human keratinocyte cell line (HaCaT) were analysed. It was demonstrated that the concentration of PVP used in this model (5% w/w) was found to be biocompatible, with cytotoxicity only occurring at concentrations as high as 15 %.
The alternate designs employed by Rabiei et al. [19] and Zhu et al. [20] highlight the flexibility of the MN design. The convenience of requiring weekly administration afforded by the design of Rabiei et al. [19] may be preferred by some patients. However, for patients who lead active lifestyles or would prefer no visible patch, the MN patch designed by Zhu et al. [20] may be preferred.
3.3. Ocular treatments
MN devices have also been developed for ocular treatment. Roy et al. [4] developed a MN patch resembling a contact lens to create a more effective method of delivering medication to the ocular field (Fig. 3a). The design consisted of dissolvable MN containing triamcinolone, a drug injected intravitreally to reduce swelling [39]. The MNs were completely dissolved within one minute of application, and in comparison to conventional eyedrops, better diffusion was reported with the MN patch [4]. When tested on live rabbits, some redness was detected within the first hour, however by four hours post-procedure, ocular health was restored to pre-operative conditions.
 Fig. 3
Fig. 3Ocular angiogenesis poses a clinical challenge in managing glaucoma; thus, finding a minimally invasive and pain-free method of reducing and controlling it could be critical for treating the condition [40]. Angiogenesis is common when there is an injury to the eye, and treatment with medications such as sunitinib malate can help to reduce the symptoms. Sunitinib malate is a multi-targeted receptor tyrosine kinase inhibitor which is administered through intravitreal injection [41]. As many MN patches require a certain level of force for the needles to penetrate, application to the eye can be problematic. For this reason, Song et al. [26] designed an application device utilising a single MN. This MN was moulded to the end of a spring-loaded applicator to allow delivery to the cornea (Fig. 3b). As MN are shorter than the depth of the cornea, the risk of corneal injury is eliminated when utilising this device. When sunitinib malate was applied to the end of the MN and injected into the cornea of C57BL/6 J mice, angiogenesis was significantly reduced compared to the same application with a 30-gauge needle. Although this device used a single MN, there is potential for an adaptation to involve multiple needles should medication need to be dispersed evenly throughout the cornea. While eye drops are still the treatment of choice for superficial injuries, Roy et al. [4] demonstrated reduced scleral damage when using a MN patch, compared to intravitreal injection. This suggests that the application of medication for deep ocular injuries with a MN patch is superior to conventional methods and has great potential for clinical use.
3.4. Anti-cancer chemotherapy
Anti-cancer drugs are known to have substantial and, at times, detrimental systemic side effects. The development of targeted treatments that can minimise unwanted off-target effects is therefore a priority. MN technology is proving effective in this field.
Pancreatic cancer is commonly treated using gemcitabine, however, as many pancreatic cancers are found at an advanced stage, the treatment often results in low success [42,43]. Fu et al. [3] developed a hydrogel patch containing gemcitabine with MNs interspersed by ‘suction cups’. This was tested on male athymic nude mice, and it was demonstrated that there was almost a 75 % increase in tumour inhibition when gemcitabine was delivered by a MN patch compared to an intraperitoneal injection. The treatment decreased systemic side effects, and the adhesive properties of this design were further tested on the porcine pancreas. Significantly improved adhesion was demonstrated compared to conventional MN patches on the irregular surface, suggesting the possibility of in vivo applications. While this proof of principle design was not clinically viable due to weekly MN replacement, it has the potential to be adapted and further designed to effectively deliver targeted cancer therapies. Moreover, this novel design has the potential to aid in a broad manner of clinical applications, including general surgeries, such as hernia repairs and wound closures.
The efficacy of MN patches has also been tested in other cancers. Breast cancer is the most common cancer detected in women, with over two million new cases diagnosed worldwide in 2018 [44]. The combination of docetaxel and doxorubicin is a treatment commonly used for breast cancers; however, these chemotherapy drugs illicit many systemic side effects [45]. Bhatnagar et al. [27] developed a MN patch containing doxorubicin and docetaxel and found it to be safer than an intra-tumoural injection when tested on a murine model (female athymic nude mice). When the triple-negative breast cancer cell line (4T1) tumours were injected directly with docetaxel, there was a sudden weight loss in the mice; a side effect not found when using the MN patch loaded with docetaxel. The study noted increased survival of mice treated with MN patches compared to the intra-tumoural injection. The slow-release nature of the dissolving MNs, as opposed to the drug being immediately available with the injection, is the likely reason for this alteration in survivability. This supports the idea that a MN application can result in a targeted treatment eliciting strong effects at the required site with decreased systemic side effects.
A limitation of these studies is that the amount of drug required for testing on murine models is significantly less than what would be required for treating humans. While targeted treatment is likely to reduce the required amount of drug, this has not yet been tested. This knowledge gap is yet to be addressed and is the work of further studies. The development of MN patches for use in delivering anti-cancer drugs has the potential to revolutionise cancer treatments by ensuring the medication is delivered more closely or directly (for skin cancers) to the tumour site, rather than systemically.
Anti-cancer drugs have also been used to reduce the occurrence of post-surgical interventions such as neointima hyperplasia. Neointima hyperplasia occurs primarily after a bypass graft has been completed and the vessel undergoes remodelling [46]. Anti-cancer drugs, such as paclitaxel, are often used to reduce the occurrence, however, the conventional application is non-biodegradable, meaning it remains in the body indefinitely. The development of biodegradable MN patches could potentially improve this delivery approach. Lee et al. [28] produced a MN cuff with solid needles coated in paclitaxel to reduce the proliferation of the tunica intima when stent surgeries or atrioventricular graft surgeries are required. The addition of paclitaxel MNs to the cuff was found to have 200 times higher delivery efficacy to tissue than other cuff-only devices and resulted in significantly less neointima hyperplasia at both two- and four-weeks post-procedure [28].
3.5. Antithrombotic medication
The use of antithrombotic medication is common after certain surgeries or for use in patients with an elevated risk of thrombosis [47]. The current administration is commonly a subcutaneous injection, often resulting in large areas of bruising on patients [48]. Innovative technologies have allowed an antithrombotic medication, hirudin, to be delivered to patients via a MN patch with minimal pain.
Men et al. [29] developed a removable MN patch coated in hirudin. When applied to the skin, it was found that peak concentrations occurred one hour after application, which was double the time it took for the conventional subcutaneous injection to reach its peak. When tested in vivo in ICR mice (Swiss albino mice), patches containing 3 mg/kg of hirudin resulted in the same protective effect that the conventional subcutaneous injection afforded. Additionally, it was noted that the bioavailability of the patch was 52.39 %, and it remained stable at −20 °C for three months.
Wu et al. [5] also demonstrated the development of a dissolving MN patch which successfully delivered hirudin to murine models (C57BL/6), emulating pulmonary embolism and artery thrombosis. In both models, the hirudin-loaded patch reduced thrombosis at a similar rate as the subcutaneous injection. The mice suffered a significant haemorrhage when hirudin was delivered through subcutaneous injection which was not evident when delivered through the MN patch. These studies present that delivery of antithrombotic medication via MN patch has a more desirable outcome than the current conventional methods.
4. ISF sampling and monitoring
Traditionally, the presence of disease and dysfunction in the body has been evaluated by obtaining samples from a tissue biopsy or via a blood test. The discovery that ISF contains clinically relevant biomarkers has resulted in the development of many new sampling methods (Table 2). Commercially, glucose monitoring has been approved to assist in managing diabetes; however, these are generally a single needle, yet the use of a microneedle patch in ISF sampling for monitoring of other components of ISF has been limited. Recent technological advancements allow a combination of microneedle patches with monitoring technology and detection of ISF components for the first time. Ultimately the development of MN patches to sample ISF is paving the way for improved monitoring and control of many clinically relevant disorders and diseases.
| Clinical Application | Design | Model | Benefits compared to conventional methods | Source |
|---|---|---|---|---|
| Diabetes management |
|
|
|
|
| Parkinsons disease |
|
|
|
|
| Ultrasensitive detection of biomarkers |
|
|
|
|
4.1. Diabetes management
Dervisevic et al. [49] developed a MN array from silicone needles coated in gold particles that could detect glucose levels in ISF. The MN array consisted of three electrodes (working, reference and counter) that accurately detect glucose concentrations in a murine model. The working range was between one and nine millimolars (mM), with a detection limit of 0.66 mM. This reference range would be sufficient as normal glucose levels in ISF are between 3.6 mM and 6 mM [54]. As this was only tested on three mice, statistical significance could not be determined; however, the limited data demonstrated a correspondence between the levels measured by the electrodes and a commercially available glucose monitor.
4.2. Parkinson's disease management
Parkinson's disease is a debilitating neurodegenerative disease [55]. Common medications prescribed include apomorphine, a non-selective dopamine agonist, and levodopa, a dopamine precursor. The dosage of these drugs is adjusted in response to the patient's symptoms [56]. Due to the neurodegenerative nature of the disease, many patients struggle to accurately describe the symptoms they are experiencing, making precise dosing a challenge for healthcare providers. High-performance liquid chromatography (HPLC) can test the plasma levels of these drugs; however, this method is costly and takes considerable time [57]. Real-time monitoring via a MN patch would benefit many patients and their carers.
Goud et al. [50,51] developed a patch that can detect the levels of apomorphine and levodopa, respectively, using a hollow MN patch. The sensor detected clinically relevant concentrations of apomorphine and levodopa in artificial ISF in a skin-mimicked environment. While these studies were not conducted in vivo, there is a promise to develop devices such as these for patients diagnosed with Parkinson's or other chronic neurodegenerative diseases.
The development of wearable devices incorporating MN patches with technological advancements could result in a more streamlined process for managing conditions such as Parkinson's disease. Healthcare workers could attach the device and monitor drug levels within patients from a central location, saving time, and reducing patient anxiety and potential non-compliance.
4.3. Ultrasensitive detection of biomarkers
MN technology is also being developed in other applications where conventional methods have significant room for improvement. A bi-layer MN patch developed by Wang et al. [52] was demonstrated in a murine model (C57BL/6) to allow the detection of proteins such as periostin in ISF using fluorophore-linked immunosorbent assay (FLISA). This improved method allowed for the detection of biomarkers in real time while not requiring the extraction of ISF. The solid bi-layer MN patch was coated with primary antibodies before insertion, followed by the addition of a secondary antibody and nano label after the patch was removed. It was shown that the patch could detect periostin from the calvaria periosteum of mice while allowing resampling and longer-term analysis of potential treatments. Additionally, FLISA is a highly specific method that can detect limited analytes on the MN more accurately than an enzyme-linked immunosorbent assay (ELISA) system [52].
Hydrogel-based MNs have also been used to sample ISF, with the fluid being used for downstream high-performance liquid chromatography (HPLC) [53]. Target drugs of isoniazid and theophylline were successfully extracted from an ex vivo skin model. This model could prove beneficial for therapeutic drug monitoring within patients, especially those who are averse to blood-based monitoring.
While the advancement in the field seems promising, many obstacles exist before the technology can be used as a point-of-care assay. For example, the relationship between protein levels in tissue compared to ISF is unknown for all proteins. Any protein used in this assay needs to be independently studied to identify an accurate reference range. Additionally, there is some removal of the target proteins from the friction caused by the MNs, and the implications still need to be investigated. Compared to the conventional methods, which result in sacrificing the testing animals, the MN approach is advantageous as it allows continued monitoring. This could also decrease the number of animals required for in vivo studies as they would not need to be sacrificed at each time point.
5. Synchronous sampling, monitoring, and delivery of drugs
The development of synchronous systems for both drug delivery and monitoring would be not only convenient but also cost-effective for the healthcare industry. If patients and healthcare workers do not monitor levels of drugs or hormones within the body regularly, there would likely be a significant increase in disease management non-compliance.
5.1. Diabetes management
Closed-loop insulin pumps have been developed and are currently used extensively by patients with diabetes. These consist of a small pod containing an insulin reservoir and a single cannula inserted into subcutaneous tissue. While this design is superior to the pinprick technique mentioned earlier, it does not offer continuous blood glucose monitoring and, therefore, still relies on patient compliance [61].
The development of a system that synchronously monitors glucose ISF and delivers insulin as required was developed by Li et al. [8]. This design consisted of a porous MN patch connected to electrodes. Following an increase in glucose, insulin was administered, and the monitor reflected the changes in glucose concentrations. In combination with the monitoring device, iontophoresis was found to have more favourable effects than the patch alone. Other studies have also employed a three-electrode system to monitor and/or control blood glucose concentrations with promising results [[58], [59], [60]].
Wu et al. [17] employed an alternate design that utilised MN patches with glucose-responsive insulin contained within the needles [17,62]. As the glucose-responsive insulin in the needles detected an increase in ISF glucose, insulin was slowly activated and released. Wu et al. [17] utilised a unique manufacturing process, printing a substrate, followed by cylinders on the base with sodium alginate and hydroxyapatite bio-ink, combined with glucose-responsive insulin. The cylinders were then stretched upwards to create the needle shape (Fig. 4). Using a type 1 diabetic murine model (male, C57BL/6), Wu et al. [17] demonstrated that the patches could maintain glucose for 20 h without intervention. Hsu et al. [63] used a MN patch containing insulin-loaded glucose-responsive nanovesicles. This patch responded to hyperglycaemia in a murine model (male Sprague-Dawley rats) and reported a healthy glucose level for 13 h [63].
 Fig. 4
Fig. 4It is important to note that these studies have been completed on murine models, and for this to be applicable for human use, the doses would need to be increased. The necessary changes in MN design to accommodate bench-to-bedside transition and the associated implications regarding accuracy and efficacy are yet to be investigated and are the work of future studies.
The approach by Wu et al. [17] and Hsu et al. [63] may prove useful in aiding non-compliant patients, as opposed to the three-electrode system employed by Dervisevic et al. [49] and Li et al. [8], as they do not require any monitoring by the patient. In addition, the wireless transmission of glucose levels may not be beneficial for non-compliant patients who may not utilise the function or for the elderly who may not be able to use the technology. However, these approaches will be beneficial for most patients (Table 3).
| Clinical Application | Design | Model | Benefits compared to conventional methods | Source |
|---|---|---|---|---|
| Diabetes management |
|
|
|
|
6. Skin and tissue biopsy
Conventional biopsy measures include needle, punch, and shave, depending on the amount of tissue required and suspected pathophysiology. The recommendation for all biopsy procedures is to numb the area with a lidocaine injection, followed by the procedure [64]. Conventional needle biopsies use a 6-gauge needle and often require stitches which can cause transient or long-term scarring and risks of infection [65].
Kislevitz et al. [6] demonstrated that a 23-gauge MN, significantly smaller than conventional biopsy needles, removed facial tissue without scaring. Sufficient tissue was obtained to perform clinical analytic techniques, including histology, electron microscopy and gene analysis. These results were comparable to the clinical information obtained from conventional biopsy samples. As this technique requires a smaller amount of tissue and reduced pain, it has the potential to become the technique of choice for biopsies.
Pozner et al. [7] also demonstrated that scarless removal of tissue was possible when using a MN on both the face (22–25 gauge) and abdomen (19–24 gauge). Pain scores at the time of removal were 0.4 ± 0.9/10 and 2.8 ± 1.1/10, respectively, suggesting an extremely low pain level. There was no scarring seven days post-procedure, and histologic analysis at day 90 revealed no residual scarring. This method required a topical cream anaesthetic instead of lidocaine injection, while the study completed by Kislevitz et al. [6] only needed ice before the procedure.
Collectively, these studies suggest that the MN method results in reduced use of anaesthetics and a reduction in pain compared to conventional techniques while obtaining sufficient tissue for clinical analyses. In addition, it shows proof of concept that MNs can result in pain-free procedures and scar-free healing without the need for stitches. While this development is promising, the microneedles would need to be long enough to penetrate to the tissue level that needed biopsy, an engineering control requiring further investigation.
| Clinical application | Design | Model | Benefits compared to conventional methods | Source |
|---|---|---|---|---|
| Skin Biopsy |
|
|
|
|
Another potential use for this technology is identifying molecular changes throughout a tissue sample with less pain and reduced scarring. Currently, when transdermal biopsies are performed, the whole tissue is tested for genomic or proteomic changes. One disadvantage of using a single MN is that it may not capture the heterogeneity in many diseases. As illustrated in Fig. 5, multiple genetic changes can be present in a single skin lesion such as melanoma, with different sections of the lesion having altered expression [66]. A clinically viable biopsy method would need to capture this diversity, suggesting multiple sample sites may be required; whether this can be achieved with a larger MN patch or multiple biopsies is yet to be determined. The benefit of MNs on a patch is that they can be used to obtain multiple small biopsies capturing this diversity.
 Fig. 5
Fig. 5Most MN patch studies investigate drug delivery or ISF monitoring, with a major gap in skin biopsies. This gap in the literature needs to be addressed as it is a vital procedure that requires developing techniques resulting in minimal pain and scarring. Should this development be realised, biopsies of sensitive areas could be less daunting and result in more frequent follow-ups of chronic skin conditions.
7. Conclusion and future perspectives
The development of MN patches has many potential uses, including reduced pain and penetration of blood vessels, increased precision in drug delivery, and improved treatment and vaccine compliance through convenient (and potentially less expensive) drug delivery. Further research is required before commercialising patches that have only been tested in pre-clinical animal models. The safety of dissolving patches and any concentration-dependent cytotoxicity is one aspect that requires more research before experimental MN patches progress to clinical trials. The minimally invasive nature of MN patches compared to conventional methods results in drug delivery with minimal pain due to the MNs not reaching the nerve bed. Compared to conventional routine testing methods, continuous monitoring of ISF biomarkers using MN patches could improve disease control as medication could be adjusted in real time with minimal bleeding. Finally, synchronous drug delivery and ISF monitoring could significantly increase medication compliance and improve the health of the patient. A major gap in the literature is the use of MN patches in tissue biopsy applications. Single MNs have been tested; however, using a MN patch to capture heterogeneity throughout a human skin tissue biopsy has not yet been reported in the literature. The development of microneedle patches which have the capacity to complete tissue biopsy would be a major step forward in disease monitoring and diagnosis. Decreased patient apprehension around biopsies could be realised with the use of microneedles due to the reduced pain and scarring. This review has established that MN patches have a strong future in the healthcare industry, particularly for diabetes management. Further studies, specifically human clinical trials, are required to establish the efficacy of MN patches for further use.
CRediT authorship contribution statement
Alissa Reinke: Writing – original draft, Investigation. Eliza J Whiteside:Conceptualization, Project administration, Supervision, Writing – review & editing. Louisa Windus: Investigation, Supervision, Writing – review & editing. Devang Desai: Writing – review & editing. Emma Stehr: Writing – review & editing. Zahra Faraji Rad: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgements
The project was funded by the University of Southern Queensland funding grant, Health, Engineering and Sciences Collaboration Grant (University of Southern Queensland) and the Toowoomba Hospital Foundation.
Data availability
-
No data was used for the research described in the article.
© 2024 The Authors. Published by Elsevier Inc.