1. Introduction
Hydroxyapatite (HA) ceramics have been widely used as a biomaterial, e.g.implant, and are produced from organic or inorganic materials. HA ceramics produced from organic materials are preferred due to its close chemical content and physical properties. It was demonstrated that HA ceramics produced from natural sources show no cellular toxicity, no fibrous tissue generation around the healing area. Also, inflammatory and pyrogenetic responses are missing in and around the transplant area [[1], [2], [3]].
Commercial CaP ceramics are mainly prepared from synthetic raw materials using several methods including crystal growth under hydrothermal conditions [[4], [5], [6], [7]], microwave irradiation [8, 9], layer hydrolysis of other calcium phosphate salts [10, 11], mechanochemical synthesis [[12], [13], [14]], plasma techniques [15, 16], solid-state reactions [[17], [18], [19], [20]], and sol-gel processing [[21], [22], [23]]. However, generally expensive reagent grade chemicals are used in the preparation of synthetic calcium phosphate ceramics coupled with complex and time-consuming techniques [[24], [25], [26]]. HA and tricalcium phosphate (TCP) can also be extracted from natural sources simply and economically with various environmental benefits [27]. Antler, tooth and bones of various natural animal sources have been used for the production of CaP ceramics including bovine [[28], [29], [30], [31]], crocodile [32], chicken [25], cow [33], deer [34], goat [35, 36], Meleagris gallopova [37], sheep [24, 38] and pig [33, 39]. Among natural marine sources of HA and TCP such as corals [[40], [41], [42]], clam shells [43], oyster shells [44, 45], razor shells [46], mussel shells [47], sea urchin spines [48, 49], sea snail shells [[50], [51], [52]] and sea shells [[53], [54], [55], [56]], fish bones [[57], [58], [59], [60], [61], [62]] are promising, abundant, and low-cost alternatives. The present comprehensive review evaluates the literature on the chemical composition, structural properties, and applications of calcium phosphates derived from various fish bones.
Food waste, waste disposal and by-product management are issues generating concerns for aquatic and terrestrial environments as well as economic and food security issues. [63, 64] The rapid growing fishing industry is a significant part of the food industry and roughly >91 million tons of fish and shellfish are consumed annually. [59] Several by-products, accounting about 40–50% of the total fish, are discharged as wastes during the processing [65]. Fish bones are huge fish processing by-products, usually recognized as an impracticable and a worthless waste. To the best of our knowledge, there is no consensus on any proper treatment procedure to evaluate fish bones in the market. Consequently, fish bones are generally used for the production of animal meal if an animal feed production facility is nearby, providing only a limited benefit. [59] Recently, evaluation of fish bones as a source of calcium phosphate (CaP) ceramics has been in the focus of many researchers due to its potential to take the advantage of producing a high-quality bioengineering material.
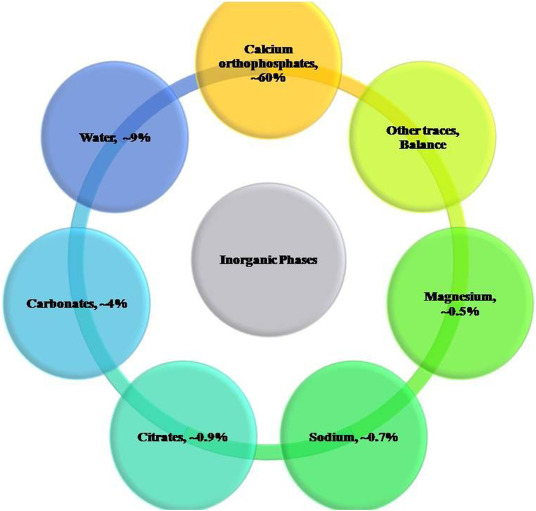
Various types of HA with different properties, that can be used for specific applications, may be synthesized from numerous sources. Depending on the crystal phase, e.g., CaP ceramics can be classified as hydroxyapatite [(Ca10(PO4)6(OH2)), Ca/P = 1.67, HA]; precipitated hydroxyapatite [Ca10−x(HPO4)x(PO4)6−x(OH)2−x, x = 1.50–1.67, pHA]; calcium deficient hydroxyapatite [Ca10−x(HPO4)x(PO4)6−x(OH)2−x, x = 1.50–1.67, CDHA]; α-tricalcium phosphate [(α-Ca3(PO4)2), Ca/P = 1.50, α-TCP]; β-tricalcium phosphate [(β-Ca3(PO4)2), Ca/P = 1.50, β-TCP]; amorphous calcium phosphate (ACP, Ca/P = 1.25–1.55); biphasic calcium phosphate[Ca3(PO4)2 + Ca10(PO4)6(OH2), Ca/P = 1.50–1.67, BCP]; dicalcium phosphate anhydrous, monetite (CaHPO4, Ca/P = 1.00, DCPA); dicalcium phosphate dihydrous, brushite (CaHPO4.2H2O, Ca/P = 1.00, DCPD); carbonated apatite[Ca5(PO5,CO3)2, Ca/P = 1.67, CA]; monocalcium phosphate monohydrate [Ca (H2PO4)2·H2O, Ca/P = 0.50, MCPM]; octacalcium phosphate [Ca8 H2(PO4)6.5H2O, Ca/P = 1.33, OCP] and tetracalcium phosphate [CaO. Cay (PO4)2, Ca/P = 2.0, TTPC]. Among these various CaP ceramics HA and its combination with TCP are widely used as bone substitute materials and coatings on dental implants owing to their close chemical and crystallographic structure similarity to the inorganic components of bones (Fig. 1) and teeth. [[66], [67], [68]] Furthermore, CaP ceramics are indispensable hard tissue reconstruction materials due to their excellent bioactivity, biocompatibility, non-toxicity, non-immunogenicity and non-inflammatory behavior.
 Fig. 1. Main inorganic components of bones.
Fig. 1. Main inorganic components of bones.2. Preparation and characterization of calcium phosphates from fish bones
Calcium phosphates can be prepared by using either natural organic or inorganic raw materials. Fish bone is considered as a one of the potential biological sources (Fig. 2) to produce calcium phosphates. Various fish bones from anchovy (Engraulis encrasicolus), barramundi (Lates calcarifer), carp (Cyprinus carpio), cuttlefish (Sepia officinalis), croaker (Micropogonias undulatus), cod (Gadus morhua), conger eel (Conger conger), flat fish (Heterosomota pleuronectiformes), flying fowl, greater amberjack (Seriola dumerili), mackerel (Trachurus trachurus), lizard, sardine (Sardina pilchardus), shark, sier, sea bass (Dicentrarchus labrax), sea bream (Sparus aurata), sheelavati (Roho labio, Fig. 3), swine, sword fish (Xiphias gladius), tilefish (Lopholatilus chamaeleonticeps), trigger fish (Balistoides viridescens) and tuna (Thunnus albacares) have been used as a starting material to produce HA and β-TCP as listed in Table 1. There are several methods to prepare calcium phosphates from fish bone.
 Fig. 2. Biological sources of HA and TCP.
Fig. 2. Biological sources of HA and TCP.
Fig. 3. a) Bones of sheelavati (Roho labio) fish b) heat treatment cycle.
Reprinted from [69], Copyright (2018), with permission from Elsevier.Table 1. The effect of fish bone source and calcination conditions on chemical, structural and textural properties of fish bone derived calcium phosphates.
| Source of fish bone | Calcination temperature (°C) | Calcination time (hour) | Phase composition | Ca/P ratio | Surface area (m2/g) | Particle size (nm) | Trace elements | Ref. |
|---|---|---|---|---|---|---|---|---|
| Anchovy | 600 | 3 | β-TCP | 1.20 (molar) | – | – | Na, Mg, K, Fe, Zn, Mn, Cu, Pb | [70] |
| Atlantic Bonito | 850 | 4 | HA + TCP | – | – | – | – | [71] |
| Barramundi | 1200 | 1 | HA + TCP | 1.62 | – | 55 | – | [72] |
| Carp | 600 | 3 | HA | 1.63 (molar) | – | – | Na, Mg, K, Fe, Zn, Mn, Cu, Pb | [70] |
| Cattle | 600 | 3 | HA | 1.64 (molar) | – | – | Na, Mg, K, Fe, Zn, Mn, Cu, Pb | [70] |
| Cod fish | 600 | 1 | HA | – | – | – | – | [73] |
| Cod fish | 1000 | 1 | HA + β − TCP | 1.50 (molar) | – | – | – | [74] |
| Cod fish | 900–1200 | 1 | HA + β − TCP | 1.49 (molar) | – | 300–500 | Na, Cl, F | [75] |
| Conger eel | 600 | 3 | HA + β − TCP | 1.56 (molar) | – | – | Na, Mg, K, Fe, Zn, Mn, Cu, Pb | [70] |
| Croaker | 600 | 3 | HA + β − TCP | 1.52 (molar) | – | – | Na, Mg, K, Fe, Zn, Mn, Cu, Pb | [70] |
| European sardine | 1000 | 1 | HA + β − TCP | 1.46 (molar) | – | 100–200 | Na, Cl, F | [27] |
| Flat fish | 600 | 3 | HA + β − TCP | 1.53 (molar) | – | – | Na, Mg, K, Fe, Zn, Mn, Cu, Pb | [70] |
| Flying fish | 600 | 3 | HA + β − TCP | 1.49 (molar) | – | – | Na, Mg, K,Fe,Zn,Mn,Cu,Pb | [70] |
| Fowl | 600 | 3 | HA | 1.60 (molar) | – | – | Na, Mg, K, Fe, Zn, Mn, Cu, Pb | [70] |
| Greater amberjack | 600 | 2 | HA | 1.51 (molar) | – | – | Na, Mg, K | [76] |
| Greater amberjack | 1000 | 2 | HA | 1.66 (molar) | 3.4 | – | – | [77] |
| Horse mackerel | 600 | 3 | HA | 1.60 (molar) | – | – | Na, Mg, K, Fe, Zn, Mn, Cu, Pb | [70] |
| Horse mackerel | 600 | 2 | HA + β − TCP | 1.42 (molar) | – | – | Na, Mg, K | [76] |
| Horse mackerel | 1000 | 2 | HA + β − TCP | 1.58 (molar) | 8.0 | – | – | [77] |
| Lizard fish | 600 | 3 | HA + β − TCP | 1.55 (molar) | – | – | Na, Mg, K, Fe, Zn, Mn, Cu, Pb | [70] |
| Mackerel | 600 | 3 | HA + β − TCP | 1.54 (molar) | – | – | Na, Mg, K, Fe, Zn, Mn, Cu, Pb | [70] |
| Sardine | 600 | 3 | HA + β − TCP | 1.60 (molar) | – | – | Na, Mg, K, Fe, Zn, Mn, Cu, Pb | [70] |
| Sea bass | 800 | 16 | HA + CaO | 2.13 (wt.) | – | – | – | [78] |
| Sea bream | 600, 900 | 24 | HA | – | – | – | – | [79] |
| Sea bream | 600 | 1 | HA | – | 10.5 | >30 | – | [80] |
| Sea bream | 800 | 1 | HA | 2.02 (wt.) | 4.0 | >70 | Na, Mg, K, Sr, Si | [80] |
| Sea bream | 1000 | 1 | HA | 2.00 (wt.) | 1.6 | >100 | Na, Mg, K, Sr, Si | [80] |
| Sea bream | 1300 | 1 | HA + TCP | 2.01 (wt.) | – | – | Na, Mg, K, Sr, Si | [80] |
| Sea bream | 600 | 3 | HA | 1.54 (molar) | – | – | Na, Mg, K, Fe, Zn, Mn, Cu, Pb | [70] |
| Sea bream | 800 | 1 | HA | 2.02 (wt.) | – | – | – | [80] |
| Sea bream | 1000 | 1 | HA | 2.00 (wt.) | – | – | – | [80] |
| Sea bream | 1300 | 1 | HA + β − TCP | 2.01 (wt.) | – | – | – | [80] |
| Sheelavati | 600 | – | HA | 1.63 (molar) | – | 64.5 | Mg | [69] |
| Sier | 400–900 | 2 | HA | – | – | – | – | [58] |
| Sier | 1200 | 2 | HA + TCP | – | – | – | – | [58] |
| Shark | 600 | 3 | HA | 1.60 (molar) | – | – | Na, Mg, K, Fe, Zn, Mn, Cu, Pb | [70] |
| Spanish mackerel | 600 | 3 | HA + β − TCP | 1.41 (molar) | – | – | Na, Mg, K, Fe, Zn, Mn, Cu, Pb | [70] |
| Swine | 600 | 3 | HA | 1.68 (molar) | – | – | Na, Mg, K, Fe, Zn, Mn, Cu, Pb | [70] |
| Sword fish | 600 | 12 | B type HA | 1.86 (molar) | – | 47 ± 10 | Na, Mg, K, Sr | [59] |
| Sword fish | 950 | 12 | HA/Beta − TCP | 1.84 (molar) | – | 66 ± 16 | Na, Mg, K, Sr | [59] |
| Tilefish | 600 | 3 | HA + β − TCP | 1.47 (molar) | – | – | Na, Mg, K, Fe, Zn, Mn, Cu, Pb | [70] |
| Thickness Perch | 900 | 3 | HA | – | – | 50–80 | – | [81] |
| Triggerfish | 600 | 3 | HA + β − TCP | 1.49 (molar) | – | – | Na, Mg, K, Fe, Zn, Mn, Cu, Pb | [70] |
| Tuna | 600 | 2 | HA | 1.55 (molar) | 19 | – | Na, Mg, K | [76] |
| Tuna | 1000 | 2 | HA + CaO | 1.71 (molar) | 3.1 | – | – | [77] |
| Tuna | 1000 | 2 | B-type carbonated HA + CaO | 1.71 (molar) | – | <1000 | – | [82] |
| Tuna | 600 | 5 | HA | 2.04 (wt.) | – | 80–300 | – | [83] |
| Tuna | 900 | 5 | HA | 1.94 (wt.) | – | 300–1000 | – | [83] |
| Tuna | 1200 | 5 | HA | 1.99 (wt.) | – | 500–2000 | – | [83] |
| Tuna | 600 | 12 | B type HA | 1.87 (molar) | – | 54 ± 10 | Na, Mg, K, Sr | [59] |
| Tuna | 950 | 12 | HA/β − TCP | 1.89 (molar) | – | 67 ± 16 | Na, Mg, K, Sr | [59] |
| Yellow tail | 600 | 2 | HA | 1.53 (molar) | – | – | Na, Mg, K | [76] |
| Yellow tail | 1000 | 2 | HA | 1.67 (molar) | 3.3 | – | – | [77] |
2.1. Thermal calcination method
The thermal calcination route, a traditional method, can serve as a straightforward approach to produce HA and β-TCP from fish bones [60]. The calcination temperature, calcination time, extraction method, and nature of bones are among the factors affecting the final properties of calcium phosphates such as Ca:P ratio, morphology, phase purity, size distribution and surface area. A general flow diagram of calcium phosphate extraction from fish bone is given in Fig. 4.
 Fig. 4. General flow diagram of calcium phosphate extraction from fish bones.
Fig. 4. General flow diagram of calcium phosphate extraction from fish bones.Synthetic HA and β-TCP are stoichiometric materials with calcium to phosphorous molar ratio of 1.67 and 1.50, respectively [70]. The Ca/P ratio of HA and β-TCP derived from natural sources differed from stoichiometric Ca/P molar ratio depending on the availability of trace elements [84]. There are studies showing that chlorine [27, 75], copper [70, 85, 86], fluorine [27, 75], iron [70, 85, 86], magnesium [59, 69, 70, 76, 80, 85], manganese [70], potassium [59, 70, 76, 80, 85], silicon [80, 86], sulfur [86], sodium [27, 59, 70, 75, 76, 80, 85], strontium [59, 80, 76] and zinc [70, 85, 86] existed as minor components among the major components (calcium, phosphorus). Calcium phosphates with trace elements could favor the cellular growth, osteointegration and enhance the biocompatibility of the material [75, 87]. The increase of trace element contents results in the reduction of the Ca/P ratio. Thus controlling of process variables has a significant effect on the preparation of high-quality stoichiometric HA and β-TCP ceramics.
Researches have been carried out various studies by using the different source of fish bones in order to determine the optimum calcination temperature [27, 59, 75, 76, 81]. Table 1 presents a brief summary of studies about the properties of various fish bone based materials obtained at several calcination conditions.
Piccirillo et al. [75] have examined the annealing of cod fish bones at various temperatures as 900, 950, 1000, 1100 and 1200 °C. The final products were composed of HA and β-TCP. The β-TCP content of the sample increased with the increased annealing temperature. The Ca/P of the samples was reported to be 1.49 which was lower than the theoretical value (1.67) explaining the formation of β-TCP containing materials. In a similar study, the bi-phasic materials containing HA and β-TCP derived from European sardine (Sardina pilchardus) bones at several annealing temperatures (600–1000 °C) and the same phenomenon was observed for the progressive conversion of HA to β-TCP with increasing temperatures [27]. The HA content of material decreased from 85.8 to 56.2 wt%, while the β-TCP increased from 14.2 to 43.8 wt% when the temperature increased from 600 to 1000 °C.
Recently, a research group has reported the extraction of hydroxyapatite from Thickness Perch bones at different temperatures (800–1050 °C) [81]. X-ray diffraction (XRD) spectra of bones treated at 800–1050 °C matched perfectly with the standard XRD pattern of crystalline HA phase (JCPDS 76-0694). XRD analysis confirmed that the crystallinity of HA increased with the increase in calcination temperature up to 950 °C as stated in another study [59].
The calcination temperature is effective on the conversion of phases. In the study of Ozawa and Suzuki [80], XRD pattern confirmed the phase transformation of HA to TCP at a temperature of 1300 °C, while pure HA was formed at 800–1200 °C temperature range.
Goto and Sasaki [76] have also investigated the effect of temperature (400–1000 °C) on the calcination of bones of tuna, yellow tail, greater amberjack, and horse mackerel fishes from Japan. HA derived from tuna, yellow tail, greater amberjack fishes bone revealed that 600 °C calcination temperature was adequate to obtain B type carbonated HA; however, the XRD results of the sample derived from Horse mackerel showed the presence of β-TCP (whitlockite; PDF no. 00-009-0169), as well as less crystalline HA. The treatment of Tuna bones at 800–1000 °C resulted in the formation of a secondary phase, CaO (PDF no. 01-070-5490) by decomposition of highly crystalline HA. Similar results were reported by Sasaki and Goto [77] for tuna bone derived samples. In addition, it was determined that the crystallitediameters of all samples were increased while the specific surface area of them decreased with the increment of calcination temperature [76]. Ozawa and Suzuki [80] also reported that the surface area and pore volume of Japanese sea bream based HA decreased from 10.5 to 1.6 m2/g and 0.28 to 0.02 mL/g when the calcination temperature increased from 600 to 1000 °C.
Bones of sheelavati (Roho labio) fish obtained from India river have been used to produce HA at different temperatures (600–1000 °C) [69]. TEM observations of HA powders indicated that the crystal sizes were increased with the increment of temperature due to the formation of large crystals by fusing together (Fig. 5). The average crystal sizes were found to be 64.5, 139 and 330 nm when the temperature was 600°, 800° and 1000 °C, respectively.

Fig. 5. TEM images of the HA samples obtained from sheelavati (Roho labio) fish a) calcined at 600 °C, b) corresponding selected area electron diffraction pattern, c) calcined at 800 °C and d) calcined at 1000 °C.
Reprinted from [69], Copyright (2018), with permission from Elsevier.Preparation of HA powders from sword fish (Xiphias gladius) and tuna (Thunnus thynnus) bones by treating the bones at 600° or 950 °C was carried out in the literature [59]. The Ca/P ratios of powders were in the range of 1.84–1.87 which was higher than the stoichiometric ratio. The crystal sizes of tuna bone based samples were slightly higher than the sword fish bone based ones (Table 1). XRD patterns confirmed the presence of B-type HA after heated at 600 °C and the existence of both B-type HA and β-TCP after heated at 950 °C.
Bioceramic production from “Atlantic Bonito” (Sarda sarda) waste bones was performed by Gunduz et al. [71] by calcination at 850 °C for 4 h. XRD patterns revealed that the final product contains HA and TCP with 66.7 and 33.3%, respectively. According to the SEM images, natural rounded porous structures with 50–120 μm porosities were determined on the surface of the ceramic. The results showed that it is possible to fabricate porous bioceramic materials using a very simple, economic and sustainable method.
Although the influence of calcination temperature on the production of HA from natural bone has previously been proposed, Coelho et al. [85] investigated the structural characterization of a nano structured HA powder obtained from the bones of Brazilian river fishes as a function of calcination and milling time. The pintado (Pseudoplatystoma corruscans), jaú (Paulicea lutkeni), and cachara (Pseudoplatystoma fasciatum) bones were calcined at 900 °C for 4, 6, 8, 10, or 12 h, and then milled for 2, 4, 8 or 16 h. It was suggested that 8 h of calcination is suitable to produce stabilized and nano structured HA powders with the Ca/P ratio of 1.64. The milling studies demonstrated that the particle size of the HA samples decreased with increased time. After 2 h of milling, the product was composed of irregular particles with size up to 1 μm. When the milling time prolonged to 4–8 h spherical particles formed with size up to 500 nm. Regular and spherical HA particles with a size of 300 nm occurred when the milling time was chosen as 16 h.
Type of fish is also effecting the composition of the final product. Hamada et al. [70] extracted calcium phosphates in three form (HA, TCP, HA + TCP) from various fishes at the same calcination temperature (600 °C) and time (3 h) with varying Ca/P ratio in the range of 1.20–1.68 (Table 1).
In tissue engineering applications, average pore size is one of the important characteristics of the scaffold. [88] The pore size should not be too small or too big. Very small pore sizes (<100 μm) avoid migration of cells, successful diffusion of essential nutrients and removal of waste products. [88, 89] Contrarily, the cell attachment is limited when the pore sizes are too big which cause a reduction in specific surface area [88]. Unfortunately, porosity also has a significant negative effect on the mechanical properties of the materials. On the other hand, the interconnecting pores of scaffolds must be permeable to facilitate events being necessary for new bone formation such as cell growth, migration and nutrient flow [88]. Therefore, the size of the interpore connections instead of the pore size might be the initial limiting factor [90]. In the literature there are conflicting results on the optimal pore size of bioceramics for bone tissue engineering applications. It is clear from Table 1that the type of raw material and calcination conditions had a remarkable effect on the properties, especially pore sizes, of the final product. Bioceramics obtained from several fish bones had pore sizes ranging from 47 to 2000 nm. Accordingly, detailed studies are necessary in designing fish bone based biomaterials by means of pore size and surface area.
In addition, the calcination temperature also affects the color of the final product due to the availability of organic residues and other elements. Grayish or black colored HA produced at lower calcination temperatures (600 °C), while white HA produced at higher calcination temperatures (>800 °C) [80]. The calcination of tuna (Thunnus obesus) light yellow bone at several temperatures resulted in four different colored products [83]. The bones calcined at 200–300 °C were dark in color (black) while the color got lighter (tanish) at 400–500 °C. The color of calcined bones turned to off-white and white when the temperatures were in the range of 600–700 and 800–1200 °C, respectively [83]. Interestingly, the calcined (850 °C, 4 h) rib bones of turbot (Psetta maxima) had a light blue color which indicated the existence of trace element manganese [91]. Benjakul et al. [92] compared the colors of ball milled (200 rpm, 2.5 h) bone raw and calcined (900 °C, 6 h) powder obtained from precooked skipjack tuna. The study demonstrated that raw biocalcium powder had higher yellowness (b*) with slightly lower lightness (L*) values than the calcined fish bone. The calcined powder was found to be whiter in color which was resulted by the removal of organic matter (amino acids, lipids, and proteins), occurred during calcination. [92]
Thermogravimetric analysis (TG)-differential thermal analysis (DTA) studies of fish bones showed that there are generally three stages of decomposition behavior. At the first, second and third decomposition stages the decomposition of water molecules, and organic compounds and decarbonation occurred, respectively [76]. Thermal behavior of fish bones is summarized in Table 2. Piccirillo et al. [93] investigated the thermal behavior of sardine bones. The higher weight loss of sardine bones when compared to other fish bones indicated that the organic matter content and/or carbonate ions in the HA lattice of sardine bones was higher.
Table 2. Thermal behavior of fish bones.
| Source of fish bone | Calcination conditions | Decomposition stage | Temperature (°C) | Weight loss (%) | Ref. |
|---|---|---|---|---|---|
| Tuna, yellow tail, Greater amberjack, horse mackerel |
Temperature: 25–1000 °C Heating rate: 20 °C/min Air flow rate: 10cm3/min |
First stage | 100–300 | 30 | [76] |
| Second stage | 300–600 | 30–40 | |||
| Third stage | >600 | 1–2 | |||
| Cod | – | First stage | <200 | 40 (total weight loss) | [75] |
| Second stage | 200–500 | ||||
| Third stage | >850 | ||||
| European sardine | – | First stage | <200 | 15 | [93] |
| Second stage | 200–600 | 30 | |||
| Third stage | 800–1000 | 10 | |||
| Thickness Perch |
Temperature: 25–1000 °C Heating rate: 5 °C/min |
First stage | 25–350 | 5.56 | [81] |
| Second stage | 350–550 | 0.91 | |||
| Third stage | 550–1000 | 2.15 | |||
| Sea Bream |
Temperature: 30–1000 °C Heating rate: 10 °C/min under air |
First stage | 30–250 | – | [80] |
| Second stage | 250–520 | ~35 | |||
| Third stage | 380–520 | ||||
| Fourth stage | 650–1300 | – | |||
| Salmon | Temperature: 50–700 °C Heating rate: 10 °C/min under continuous nitrogen | First stage | 350 | ~40 (total weight loss) | [62] |
| Second stage | 463 | ||||
| Tuna | Temperature: heating rate: 10 °C/min under continuous nitrogen | First stage | 100.5 | ~35 (total weight loss) |
[83] [60] |
| Second stage | 356.6 |
Thermal properties of the Brazilian river fish bone based nano structured hydroxyapatite were characterized by Coelho et al. [94] using thermal wave interferometry and non-adiabatic relaxation calorimetry. The samples were milled for 0–32 h and then sintered at 1000 °C for 4 h in an oxidant atmosphere prior to analysis. Thermal conductivity and diffusivity results showed that the thermo-physical parameters increase with milling time. However, samples presented a transition in the plateau-like interval from 8 to 16 h. The crystallites reduced from 80 nm to near 22 nm when milling time was increased from 0 to 16 h. Thanks to the changes in HA microstructure, both parameters continue to increase after 16 h. The results of the study are very significant for modeling any heat transportation in HA-based material applied in replacing or restoring of bone in surgical operations.
The scanning electron microscopy (SEM) and transmission electron microscopy (TEM) observations have shown that fish bone based materials were composed of particles including dandelion-like [95], elongated [93], rod-like [59, 75, 76, 78], needle-like and sphere [81, 85] shaped crystals. A representative scheme for the common morphologies for fish bone derived HA is given in Fig. 6. There are some reports about the relation between the geometrical shape of particles and mechanical properties, bioactivity and osteogenic potential of HA and HA based composites [[96], [97], [98]]. Additionally, it was observed that particle size can influence the biological behavior of these materials. [[99], [100], [101]] The results showed that the size of the HA nano particles were effective on cell proliferation and cell apoptosis [99]. It was found that HA with small diameters (∼20 nm) were better than bigger particles (80 nm and micro-sized) for cell growth promotion and cell apoptosis inhibition of osteoblast-like cells [99]. Consequently, the biological response of HA is very complicated. Further studies to investigate the effect of particle shape/size on the in vivo and in vitrobehaviors (e.g. bioactivity, biocompatibility, degradability and osteoconductivity) of fish bone based bioceramics will be rewarding for the bone tissue substitution, design of artificial organs and drug delivery agents.
 Fig. 6. A representative scheme of the common morphologies for fish bone derived HA particles.
Fig. 6. A representative scheme of the common morphologies for fish bone derived HA particles.2.2. Alternative preparation methods
Researchers have conducted studies on the calcium phosphates production with alternative methods such as alkaline hydrolysis, hydrothermal and laser ablation (Fig. 7). Synthesis of blue shark (Prionace glauca) fish bone based microscale particles by using laser ablation system combined with a compressed gas jet without previous calcination was reported by Boutinguiza et al. [102]. The extracted micro and nanoscale particles were composed of HA whitlockite (Ca3(PO4)2) and HA. The study showed that it is possible to obtain calcium phosphates by direct ablation method without calcination.