1. Introduction
1.1. Overview
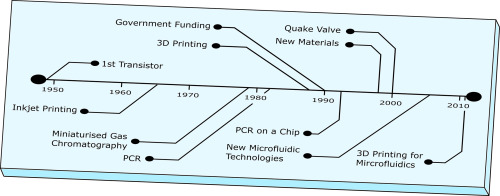
Microfluidics has often been heralded as a game changer in life sciences research and industry [1]. However, despite a great deal of work over the last few decades, it has not been the harbinger for scientific advancement that it was initially predicted to be and is now more commonly referred to as a discipline in “adolescence” [2]. Microfluidics, that is systems with a width/height scale between 100 nm and 100 μm [3], is a field that has seen a great deal of research in recent times with many devices now capable of outperforming their classical ancestors as well as the development of new devices that have allowed for novel functionality and the study of phenomena that were elusive to macroscale devices. In this review, we look back at the developments that have had the greatest impact on microfluidics with many of the key advances summarised in Fig. 1. To begin with, we explain the physics of fluids on the microscale to understand the effects that dominate the behaviour of liquids and mixtures. These effects explain many of the advantages of microfluidics such as faster reaction times and simple kinematics. We then look at the origins of microfluidics in the microelectronics industry and look at how this informed the manufacture of early devices before new technologies such as replica moulding, embossing, and injection moulding were developed and adapted to better suit the needs of the growing field. Manufacture is also dictated by the choice of material. We again look at this from a historical perspective and discuss how material selection for.
 Fig. 1. Timeline highlighting the main advances in the field of microfluidics starting with the invention of the transistor leading up to the rise in 3D Printed devices.
Fig. 1. Timeline highlighting the main advances in the field of microfluidics starting with the invention of the transistor leading up to the rise in 3D Printed devices.microfluidic devices changed as new fabrication technologies became available and the requirements of microfluidic devices (such as optical transparency) meant that materials such as silicon were superseded by glass and plastics. Finally, we look at the most recent developments in the field and discuss directions for future research to ensure that microfluidics reaches its full potential.
1.2. Physics of microfluidics
To understand the full benefit of these miniaturized systems, it is important to first understand the physics of fluids on this scale and how this affects their behaviour. Firstly, the ratio of inertial forces to viscous forces in a fluidic system is described by the dimensionless Reynolds number (Re) which is given in Eq. 1[4]:(1)Here, ρ is the density of the fluid, ν is the velocity, L is the characteristic linear dimension of the system and μ is the dynamic viscosity. From this equation, it is apparent that as the characteristic dimensions of the system are reduced, the Reynold's number is also reduced. As Reynolds number falls below 2000, the system enters what is known as the laminar flow regime which carries several differences over turbulent flow (Re > 4000). Firstly, laminar flow is highly predictable meaning mathematical modelling of these systems is less intensive. Additionally, molecular transport in the laminar regime differs from the turbulent as there is no convective mixing, only diffusion, which again leads to highly predictable kinetics. In microfluidic systems, Re is almost always in the laminar flow regime. In addition to the Reynolds number, the Péclet Number(Eq. 2) [5] also gives information on the mass transport of a fluid.(2)Here D is the coefficient of diffusion, and Pe describes the ratio of advective to diffusive transport of molecules in a fluid. From Eq. 2, reducing the dimensions of a system leads to a reduction in the Peclet number. As with the Reynolds Number, this means that the kinetics of a system are more predictable. Secondly, the behaviour of a fluid's surface differs between the macro- and the microscale. Surface tension describes a fluids affinity to modify its surface to air interface to reduce its free energy. Interfacial tension describes the same phenomena but in two immiscible fluids, for example, oil in water. This phenomenon has been utilised to great effect in the field of droplet microfluidics [6] which will be discussed in section 5.1. On the microscale, these forces dominate with respect to gravity (the dominant force on the macroscale) and can be used as a method to drive fluids without the need for pumps. Thirdly, capillary forces also begin to dominate gravitational forces as the characteristic dimensions are reduced. Capillary forces describe the force on a fluid that allows it to travel through a porous material or narrow capillary. Again, at the microscale, this dominates over gravity and lead to the development of many analytical devices such as blood glucose meters and cheap pregnancy tests as well as the development of paper analytical devices (PADs) [7], also discussed in more detail in further sections. Finally, reaction times in microfluidic systems are much quicker than conventional devices. This is due to the smaller dimensions of the systems leading to a shorter diffusion time for any given molecule. An approximation for diffusion time is shown in Eq. 3 [5]:(3)Where x is the distance travelled by one molecule of solute along one axis after time, t, and D is the diffusion coefficient of the solute. From the above equation, it is apparent that:(4)Therefore, it becomes clear that as the characteristic dimensions of a system are decreased, the time taken for molecules to diffuse across said system are reduced, thus leading to faster reaction times in microfluidic devices. This becomes increasingly important when larger molecules with a lower coefficient of diffusion, such as DNA, are considered.
With the above phenomena in mind, researchers have been able to leverage these affects in devices that can perform tasks with great value to biological and chemical studies. Additionally, due to their reduced size, microfluidic systems also consume less reagents than conventional fluidic platforms making them an ideal tool when the cost of chemicals is an issue. For example, blood glucose meters only require a small drop of blood and can give a readout of blood glucose concentration in seconds allowing for patients to monitor their condition and comply to their treatment plans from their own homes. However, despite a great deal of work and possibilities, microfluidics has not caught on in the way they were initially predicted to. The promise of “lab-on-a-chip” style devices has been realised in a “chip-in-a-lab” fashion and a lack of standardisation has meant that microfluidics has, in large, remained an academic research tool. Furthermore, the disconnect between the end users and the manufacturers of these devices has meant there has been a great deal of solutionism in the design of these systems. That is, unnecessarily intricate and complicated devices have been designed and manufactured by engineers despite the device having little or no “real world” applications [2,8]. In this review, we look back at the origins and highlight the major developments in the field that have brought microfluidics to where it is today.
2. Birth of the field
Although many of the advancements in microfluidics took place towards the end of the 20th century, its origins begin in the same manner as microelectronics. Driven by a need to improve the reliability of the mechanical relay systems used in telephone lines, William Shockley, Walter Brattain and John Bardeen of the Bell Telephone Laboratories invented the transistor in 1947 [9]. Building on this work, Jay Andrus patented the technique of photoengraving, which had been used previously to create patterns for printed circuit boards, to create much finer details that allowed for semiconductor devices such as those demonstrated by Shockley, Brattain and Bardeen, to be fabricated in silicon [10]. This is the process of photolithography (shown in Fig. 2) which would later become the standard in microelectronic manufacturing. This work was furthered by Jack Kilby at Texas Instruments (patented in 1964) which details how many discrete components such as transistors, resistors and capacitors, can be manufactured in an individual silicon crystal to form an oscillator circuit [11]. This demonstration of the first ever integrated circuit (IC) brought about a revolution in microelectronics and ushered in the “silicon age” as companies sought to create smaller, more reliable consumer electronics. The impact of this was so significant that Kilby was awarded a Nobel Prize in 2000.
 Fig. 2. A typical photolithography process. Process consists of spinning a photoresist to a desired thickness on the substrate before heating to remove any solvents. Resist is then irradiated with UV light through a photomask before a post exposure bake (if required) accelerates the curing of the resist. For positive tone resits, the areas not exposed to the radiation are removed during the development while the opposite is the case for negative tone.
Fig. 2. A typical photolithography process. Process consists of spinning a photoresist to a desired thickness on the substrate before heating to remove any solvents. Resist is then irradiated with UV light through a photomask before a post exposure bake (if required) accelerates the curing of the resist. For positive tone resits, the areas not exposed to the radiation are removed during the development while the opposite is the case for negative tone.As the Silicon Valley revolution was getting underway, another new technology emerged that would have a great influence on the microfluidics industry in the future: Inkjet printing. Although first realised in a working device by Richard Sweet in 1965 [12], the mechanics behind the printer was first described by Walter Rayleigh in 1879 [13]. Rayleigh explained how a falling, continuous stream of fluid breaks up into discrete droplets to minimise their surface area (with respect to a column of the same volume), and thus surface energy. Sweet utilised this phenomena by forcing ink through a small, vibrating nozzle with a 35 μm diameter. As the ink exited the nozzle, discrete droplets were formed which were then charged by an input electrode. The droplets then fell through a uniform electric field which deflected the drops depending on their charge before they hit the paper. As the paper is moved underneath the jet, a trace of the charge of each drop with respect to time is obtained which Sweet demonstrated as an oscillograph capable of moderate frequency recording but with increased convenience and cost over optical based methods. In the design and manufacture of this oscillograph, Sweet demonstrated what can be regarded as the first microfluidic device. Further work on this technology by Bassous et al. at IBM in 1977 showed that through photolithography (a process which had become the standard for silicon manufacturing), an array of inkjet nozzles could be fabricated in an individual silicon wafer [14]. While this not only helped commercialise inkjet printers and make them more affordable and reliable for consumers, this process also illustrated that silicon could be used as a material for the mass manufacture of microfluidic devices.
As time progressed and a wider range of sensors and transducers as well as more refined photolithographic and etching techniques for silicon were developed, researchers began to turn their attention to using these techniques to solve problem outwith electronics. Molecular analysis was the first field to receive this concentration as it became obvious that the benefits of minimising the fluidic systems that were the current standard could lead to much more robust equipment.
With the phenomena associated with microfluidics in mind, work conducted at Stanford University and published in 1979 details the design and manufacture of a microscale gas chromatography system [15]. In this seminal publication, Terry et al. describe how their device, manufactured through a combination of photolithography and etching steps, consists of an injection valve and a 1.5 m long capillary coil. A thermal conductivity sensor, also manufactured with the techniques developed in the I.C. industry, was manufactured in a batch processand attached to the end of the capillary to serve as a detector. This detector, when coupled with the capillary to separate the gases in the system, could provide highly sensitive and specific analysis of the composition of the injected gas mixture. Furthermore, the paper also details how reducing the cross-sectional area of the capillary led to an increase in performance of the device – in line with the theory described in section 1.2. Additionally, the valve chosen had a dead volume of ~4 nl and was capable of injecting volumes of as small as 1 nl into the capillary showing how reducing the dimensions of a device lead to lower reagent consumption. Even though this device could be manufactured on a single 5 cm silicon wafer (seen in Fig. 3), it still had comparable performance to the much bulkier techniques that were the standard at the time. This device is now widely regarded as the first “Lab on a Chip” or “micro-Total Analysis System” although these terms had not been coined at this point. Also, it is widely regarded that this paper heralded the true birth of microfluidics as a field of its own. Indeed, even today, the gas chromatography system still holds up to the definitions of a micro-Total Analysis System even though these criteria had not been put in place at this point.
 Fig. 3. A – Photograph of Gas Chromatography System described by Terry et al. Device consists of a 1.5 m long, spiral capillary column with input (top right) and exhaust (right) for gas sample. Flow within the device is controlled with a valve (top left) before the capillary coil and the detector can be seen on the right of the device. B – Schematic showing all the fluid handling components housed on the one silicon wafer. Reprinted from reference [15].
Fig. 3. A – Photograph of Gas Chromatography System described by Terry et al. Device consists of a 1.5 m long, spiral capillary column with input (top right) and exhaust (right) for gas sample. Flow within the device is controlled with a valve (top left) before the capillary coil and the detector can be seen on the right of the device. B – Schematic showing all the fluid handling components housed on the one silicon wafer. Reprinted from reference [15].3. ‘80s: early research in microfluidics
3.1. LIGA process
LIGA (Lithographie, Galvanoformung, Abformung; German for Lithography, Electroplating, Moulding) is a process that adds an electroplating step after photolithography to create moulds that can be used to produce many replicas of the original master. After photolithography, a seed layer of nickel‑vanadium (NiV) is deposited onto the master by sputter coating and then a thicker, support layer is deposited with electroplating. This technique has been utilised for the production of masters for injection moulding biomimetic surfaces [16]. Other variations of this technique allow for the construction of three-dimensional coils with integrated cores as well as the manufacture of rotor elements [17]. DEEMO (dry etch, electroplating, moulding) exists as a further extension to the LIGA techniques and has been demonstrated as a viable method for the production of stamps and moulds for embossing [18]. However, as a method for producing metal parts, its uptake has been hampered by its reliance on expert operators and clean room facilities despite the high resolution and low feature size that can be achieved.
3.2. Valves
After Terry et al. published their work [15], it became apparent that microfluidics had the potential to allow researchers to develop new, more powerful tools for molecular analysis. With this in mind, they set about to create tools that would allow for the manipulation of fluids on the micro-scale, that is the pumps and valves that could control and manipulate fluid flow in such a way that robust devices could be created. The first type of valves that were made took the forms of diaphragms (similar to the one in the gas chromatography system described by Terry et al.). In a bid to make these valves more reliable, as they often required large pressures to actuate the moving parts, Huff et al. developed a valve that was balanced by the pressure of the fluid thus allowing the diaphragm to be manipulated by much smaller actuation forces [19]. Due to the increased effect of electrostatic forces at the microscale, the forces required to manipulate moving parts are too large meaning that the magnetic actuators (motors and solenoids) that were in use on the macroscale, were not up to the task. To further combat this, Jerman developed a diaphragm based valve that opened through the electrical stimulation of bimetallic contacts [20]. This valve was able to operate under the flow and pressure ranges that were useful for MEMS applications however, complex manufacturing meant that these kinds of flow regulators never really caught on in within the microfluidics community.
Similar to diaphragm valves, researchers also used simple cantilever structures to manufacture check valve to manipulate flow in MEMS devices. One such example of such a valve is the batch fabricated, non-reverse valve described by Tiren et al. [21]. This was a two-piece device with one piece harbouring the cantilever structure and another containing the inlet and exhaust while also sealing the chamber. As with the diaphragm valves, this design had the advantages of fast operation as well as small dead volumes. Due to the two-piece nature and thus complex manufacture of this valve, Ohnstein created a version that could be manufactured in one piece of silicon, thus simplifying the manufacturing process [22].
3.3. Pumps
Building on the valves created for silicon MEMS, researchers also wanted to miniaturise the pumps so that these too could be incorporated onto a single device. Examples of such devices come from Smits, who designed peristaltic pumps with the aim of delivering minute quantities of insulin to diabetic patients. His pump, consisting of piezoelectrically manipulated diaphragms and check valves to inhibit back flow could deliver a fluid with a rate of 100 μl/min [23]. Van Lintel also created peristaltic pumps and included a fail-safe so that there was no backflow when the pump was turned off [24]. The above gives a brief overview on some of the early work conducted in valves and pumps of MEMS in silicon substrates however, more comprehensive reviews on this subject can be found elsewhere [25].
Despite a great deal of work being done on the manufacture and operation of these valves and pumps, this technology never really found its places in the microfluidics community for a variety of reasons. Namely, the design and manufacture of such devices required a great deal of expertise that was not had by those in the field of molecular analysis meaning that the design and manufacture of these devices was out of reach for many of the end users of this technology. Additionally, these valves and pumps were all based in silicon as this material can be processed by thin films such as photoresists (surface machining) and etched (bulk machining) in processes that were already highly developed and understood to the point where three dimensional structures could be created [26]. Additionally, due to its use in the microelectronics industry, batch fabrication protocols exist for silicon devices allowing for an economy of scale. Furthermore, silicon is both thermally and chemically stable – attractive properties when microfluidic devices often require heaters and must be robust enough to carry out sensitive operations. Due to the crystalline nature of silicon, anisotropic etching can also be achieved depending on the orientation of the lattice structure – a property not present in many other materials and means that channels with predictable sidewall geometries can be created through wet etching. However, as microfluidics turned towards the life sciences, it became apparent that silicon may not be the ideal material.
3.4. 3D printing
The ‘80s also saw the invention of another technology that would have a huge impact on the microfluidics industry. Developed by Charles Hull in 1986, stereolithography (SLA) describes the process in which three dimensional objects are created through the stacking of two dimensional laminae [27]. In this method, computer software breaks down a three-dimensional model into a sequence of two dimensional layers which are then projected in series onto a build platform submerged in UV curable resin. After each layer has had time to solidify, the build platform is moved upwards and the next layer is cured. This process is repeated until the full object has been manufactured. This invention meant that researchers could create short production runs of bespoke parts without the requirement for expensive and specialist equipment and tooling. Although developed in the ‘80s, SLA would not become a commonplace fabrication technique until much later. The impact of this technology on microfluidics will be discussed in section 5.5.
4. ‘90s: microfluidics finds its feet
4.1. μ-TAS concept
With the trend towards these microscale fluidic devices, Manz et al. proposed that it would be possible to create total analysis systems (TAS) [28], that is, a system that could carry out all of the functions required for analysis: sampling, transport of the sample, any sample preparation steps including chemical reactions and separations, as well as detection. Furthermore, these functions should be carried out automatically. If the device in question had characteristic dimensions on the microscale, they would be termed “miniaturized Total Analysis System” (μTAS). In this influential publication [28], Manz et al. laid out how the physical advantages of fluid mechanics on the microscale would lead to faster, more efficient analysis. Additionally, it was hypothesised that many channels could be fabricated into a small area allowing for simultaneous analysis of multiple samples. This paper showed what could be possible with microfluidic devices and paved the way for many for the advances and technologies that are routine in labs today.
4.2. DARPA
With the birth of the μTAS concept, many other areas of science began to take an interest in microfluidic technologies and began to see what this emerging technology had to offer. One of these sectors was defense. As the Cold War was coming to an end, there was a perceived increased military and terrorist threat from biological and chemical weapons and with this in mind, the Defense Advanced Projects Research Agency (DARPA) funded a lot of microfluidics research in a bid to create portable, field deployable devices capable of detecting these weapons [2]. Importantly, this focused funding lead to an increased drive to develop functional microfluidic devices.
4.3. The human genome project
Another major motivator towards microfluidics emerged towards the beginning of the ‘90s – the Human Genome Project (HGP). Launched in 1990, the project was set up with the aim of mapping the entire human genome within 15 years and was publicly funded by the National Institute of Health (NIH) and the US Department of Energy to the tune of $3 billion [29]. As the project began, however, it became clear that current DNA sequencing technologies would not be up to such a mammoth task.
As microfluidics turned towards biological detection, researchers began to discover the many disadvantages associated with the use of silicon. Firstly, silicon is expensive. With one of the main tenets of microfluidics being that devices should be cheaper than the alternatives, the cost of silicon lessens its attractiveness as a viable material for the mass production of microfluidics. Secondly, silicon is brittle which means the devices are often delicate so consideration must be taken when parts are to be transported. Thirdly, silicon is opaque to light in the visible and ultra violet (UV) spectrum – an important factor when many sensors, such as those for DNA analysis, use light as a detection method. Finally, the protocols to bond silicon to other silicon substrates or materials requires considerable expertise and facilities [8]. Bonding is a very important aspect when microfluidic devices are considered as the majority of chips are manufactured as sandwich structures. To move away from silicon, researchers first turned their attention to glass. Glass has similar advantageous properties to silicon – that is it can be processed by thin films in much the same way as those techniques developed in the microelectronics industry. Glass also came with the advantage of being optically transparent, which allowed for light based detection methods to be incorporated into devices, thus allowing for a new generation of optic based microfluidic biosensors.
In the decades preceding the ‘90s, DNA sequencing relied on slab gel electrophoresis [30]. As the preparation of these gels was laborious and time consuming and the act of carrying out the separations difficult to automate, slab gel electrophoresis was adequate for small scale research applications but would have significantly hindered the HGP. With this in mind, researchers began to apply the principles of microfluidics towards creating a more robust method of sequencing DNA. In 1990, Swerdlow and Gesteland showed that a 75 μm diameter silica capillary filled with electrophoresis gel could be used in place of a slab gel [31]. What is more, when they compared this device to slab gel electrophoresis, they found that their capillary electrophoresis (CE) was 3× faster and had a 2.4× better resolution. These advantages were due to the fact that the thermal properties of the capillaries meant that they were less susceptible to joule heating when exposed to high electric fields. This meant that up to 50× higher electric fields could be applied to CE devices compared to their slab counterparts [30] so shorter separation distances could be used which in turn contributed to the faster operation. Due to the small size of these capillaries, it was not long before photolithography techniques were used to fabricate these channels. By 1994, both Woolley and Mathies, and Effenhauser et al. had manufactured CE arrays in planar glass substrates with the function of separating DNA fragments based on their size – the first and vital step in the sequencing of DNA [32,33]. Steps taken towards on-chip DNA sequencing were taken also by Woolley et al. who demonstrated that the sequencing of fragments with ~150 base pairs was achievable with 97% accuracy [34]. Continuing on from this work, Schmalzing et al. created a theoretical framework to help optimise the design of DNA sequencing experiments [35]. Using this, they were able to sequence fragments with 400 base pairs.
Finally, Woolley et al., and Simpson et al. demonstrated that multiple CE channels could be fabricated onto one glass chip [32,36,37]. These multiplex devices meant that DNA sequencing was faster and simpler to perform than ever before. Another key advantage of these microfluidic platforms was the economic use of reagents. All of the methods described above were capable of separating DNA from sample volumes as small as a few nano-litres.
However, during this period, it was not just DNA sequencing that benefitted from microfluidics. Polymerase chain reaction (PCR) suffered from the same drawbacks as slab gel electrophoresis which motivated Northrup et al. to develop the first chip based PCR thermocycler [38]. This device meant that it was now possible for researchers to incorporate sample preparation as well as detection and analysis into a single microfluidic device in line with the requirements set out by Manz et al. for μTAS. Furthermore, these advances meant that the HGP was completed on time in 2003 as well as contributing greatly to our understanding of microfluidics.
4.4. PDMS
As described above, glass was used as an alternative to silicon for many microfluidic applications. However, glass and the plastic poly(methyl methacrylate) (PMMA) (which was also used to make devices) were materials that were available throughout microfluidics research and hence are not considered as advances within the field. Due to this, they are not examined in detail here, although how material selection changed as microfluidics progresses is discussed in section 4.7. Furthermore, glass was superseded as the standard microfluidics material by cheaper alternatives that would allow for a simpler approach to the manufacture of microfluidics while still allowing for the incorporation of valves and pumps. Towards the late 20th century, the elastomeric material poly(dimethylsiloxane) (PDMS), pioneered by George Whitesides and his group at Harvard, quickly became the most popular material for the manufacture of microfluidic devices [39]. Compared to glass and silicon, the fabrication of devices in PDMS is simple and does not require expensive clean room facilities. Firstly, a master structure is prepared (through silicon micromachining or otherwise). Next, the PDMS base and curing agent are mixed together before the solution is poured over the mould. The low surface energy of the PDMS means that it readily flows into small features and release from the mould is simple. This in turn means that features with sub 0.1 μm dimensions can be cast with ease [40]. Another main advantage of PDMS is how it can be bonded to itself or to other materials. PDMS channels can be sealed through a variety of methods. In the simplest method, tape can be used to seal channels [41] but it is more commonplace for devices to be sealed against a glass slide or with a further layer of PDMS. When placed in conformal contact with another substrate, the elastomeric nature of PDMS means that is forms a seal capable of withstanding moderate fluid pressures [39]. Additionally, PDMS can be irreversibly bonded to other materials through the plasma treatment of the two interfaces if a high-pressure seal is required [42]. Another method of bonding is to have one side with a saturation of the base in contact with a side with a saturation of the curing agent [42]. When heated, an irreversible bond between the two sides is formed without the need for an adhesive that could otherwise clog channels. These simple processes meant that prototyping of microfluidics devices became quick and cheap hence its uptake in the microfluidic community.
Concomitant to the advantages described above, another benefit of PDMS was its soft, elastic nature [43]. This lead to Stephen Quake's group at Stanford university to develop the Quake valve which would become the most commonly used valve in the field of microfluidics [42]. Driven by the μTAS concept that every microfluidics device should be able to carry out all the necessary steps required for analysis, Quake sought to recreate the valves and pumps that had been manufactured for the silicon MEMS industry in the now popular PDMS. The valve he created was constructed of a multilayer PDMS structure although 3D printed valves have been demonstrated (Fig. 4C) [44]. One layer housed the channels for fluid flow while a control line running perpendicular to that channel was fabricated in another. When the pressure in the control line was increased, the control line bulges and deforms the channel to such an extent that the flow in the channel can be completely stymied. A schematic of this can be seen in Fig. 4. These valves also have a low dead volume and fast operation that is congruous to the requirements of μTAS. As with silicon valves, these PDMS valves can be activated in sequence to produce peristaltic pumps that come with the ease of fabrication inherent to PDMS. Examples of applications that have utilised PDMS devices include biochemicalassays [45], genomics [46], chemical reactions [47], and biological detection [48]. Many of these applications were made possible by PDMS's permeability to gases making PDMS is an ideal material for use in live cell studies [49].
 Fig. 4. A – Process of manufacture of the Quake valve. Channel is moulded in the part on the left with the pneumatic control line in the part on the right. The control line is then placed on top of the channel. During operation, an increase in pressure in the control line deforms the channel to such an extent that flow in the microfluidic channel is blocked. Reprinted from reference [43]. B shows the operation of the valve. When the valve is open, fluid can flow in the lower channel. When pressure in the control channel is increased, the flow channel is deformed and obstructs the flow. Ca – device with an array of quake valves (open) manufactured through stereolithography and closed in Cb. Reprinted from reference [44].
Fig. 4. A – Process of manufacture of the Quake valve. Channel is moulded in the part on the left with the pneumatic control line in the part on the right. The control line is then placed on top of the channel. During operation, an increase in pressure in the control line deforms the channel to such an extent that flow in the microfluidic channel is blocked. Reprinted from reference [43]. B shows the operation of the valve. When the valve is open, fluid can flow in the lower channel. When pressure in the control channel is increased, the flow channel is deformed and obstructs the flow. Ca – device with an array of quake valves (open) manufactured through stereolithography and closed in Cb. Reprinted from reference [44].Not content with simply using PDMS as a substrate for microfluidics, Whitesides also pioneered the material's use as a fabrication tool. This set of techniques, termed “Soft Lithography”, due to the soft, elastomeric nature of PDMS, is discussed briefly with respect to microfluidics however more complete reviews can be found elsewhere [[50], [51], [52]].
4.5. Advances in micro manufacturing techniques
4.5.1. Replica moulding
Perhaps the simplest of the polymer moulding practises, replica moulding involves casting a polymer against a stamp or a mould [53]. Fig. 5 shows the replica moulding process and a device replicated from a silicon master [54]. When PDMS is used as the mould material, the mould can be deformed around curved or contoured substrates to pattern areas that are elusive to photolithography [55]. Moreover, replica moulding can be used to replicate nano-scale features and the use of elastomeric stamps means that release from the mould is not an issue when compared to rigid materials [56]. The main drawback of replica moulding to produce microfluidic parts is that it is currently not an automated process, thus it is not high throughput enough for industrial scale manufacture. Although injection moulding provides a means of replica moulding with a much higher degree of throughput, this technology will be discussed in greater detail section 4.5.5 due to the wealth of literature surrounding this topic.
 Fig. 5. A – Schematic of replica moulding process. A PDMS master is prepared before a prepolymer is poured on top. Once the prepolymer as flowed into all areas of the master, the polymer is cured and the PDMS master is removed giving a replicated part. B – Microfluidic chemostat manufactured through replica moulding. Coloured dyes used to visualise the channels and coin for scale. Reprinted from reference [54].
Fig. 5. A – Schematic of replica moulding process. A PDMS master is prepared before a prepolymer is poured on top. Once the prepolymer as flowed into all areas of the master, the polymer is cured and the PDMS master is removed giving a replicated part. B – Microfluidic chemostat manufactured through replica moulding. Coloured dyes used to visualise the channels and coin for scale. Reprinted from reference [54].4.5.2. Embossing/nano imprint lithography
Embossing, or imprint lithography, is a technique that involves the patterning of a material – usually a polymer – against a mould or a stamp with a relief pattern. This can be executed in number of ways. Most commonly, the substrate is brought to 40–50 °C above its glass transition temperature (Tg) before the stamp is then brought into contact with the substrate and both parts are cooled and the polymer returns to below Tg. The moulded part is then released from the stamp. Additionally, imprint lithography can be achieved using a pre-polymer which can then be cured to give a solid pattern. In industry, compact discs (CDs) are an example of how this technique has been used in the mass production of consumer goods [57] – although many of these processes have now been switched to injection moulding. Additionally, in research settings embossing has been used to manufacture gratings capable of coupling light into waveguides [58], as well as reliably reproducing features as small as 25 nm [59] which can then be used as mask for etching or as a sacrificial layer for the lift off of metals [60]. Stamps for imprint lithography have been manufactured with a number of techniques in a number of materials, such as PMMA [61] and quartz [62]. Furthermore, companies have developed commercial systems for the creation of embossing stamps and the imprinting of substrates [63]. Embossing is also advantageous as a replication method as it requires little flow of the polymer so there are low thermal stresses in the final part [64]. While this process can be automated, the time taken to heat and cool the substrate and tool means that the cycle time is too long for high throughput fabrication.
4.5.3. SU-8
The development of the negative resist, SU-8, by IBM, lead to the design and realisation of many MEMS and lab-on-a-chip style devices that were elusive with the thin films developed for the microelectronics industry.
First described in 1998, SU-8 allowed researchers to create structures with high aspect ratio in much thicker resist layers than had been observed before (up to 1200 μm) [65]. Alongside the ability to form these thick layers, the mechanical, thermal and chemical properties that allow it to be used for the manufacture of nickel moulds for injection moulding [65], the direct manufacture of micromechanical parts such as gears [66], and thermal flow sensors manufactured directly into the photo-plastic which highlighted its suitability for microfluidics [67]. SU-8 is processed in much the same manner as other photoresists (Fig. 2) however the pre-bake and exposure times must be lengthened to compensate for the thicker layer. Additionally, a post-exposure bake is added to accelerate the cross linking of the polymer in the regions exposed to the UV radiation. SU-8 has also been used to create multi-layer structures that can be used to construct complex three-dimensional shapes. Mata et al. described a process in which 10 μm diameter posts or holes are patterned onto or around larger features with only one development step [26]. This procedure also allowed for the creation of overhanging structures that were previously only achievable with complicated, multi-step protocols involving bonding, etching and electroplating [68]. The multilayer SU-8 technique was further utilised porous scaffolds for tissue engineering while having a surface that would promote the differentiation and proliferation of cell lines. Indeed, the advent of this photoresist lead to the fabrication of microfluidics devices manufactured entirely in SU-8. Sato et al. manufactured a purely SU-8 fluidic channel with built in 3D microstructures that could generate droplets of fluid [69].
4.5.4. Rapid prototyping
Despite microelectronics' and microfluidics' reliance on photolithography, the process suffers from one main setback: the use of chromium on quartz masks to pattern the UV light onto the desired regions of resist. Not only are these masks expensive (~$400 per mask), they are also time consuming and require considerable expertise to manufacture – a barrier to the uptake of this technique in biology and chemistry. In order to combat this issue, Qin et al. printed patterns onto acetate films that could be used in place of the quartz masks [70]. Here, a standard laser imaging system is used to pattern black ink onto the films in the areas that are not to be exposed. This technique allows for the rapid production of photolithography masks at a fraction of the cost (~$1 per square inch) and time (~2 h from design to manufacture) of their quartz counterparts. Additionally, the mechanical flexibility of these masks means that non-planar surfaces can be manufactured. While these masks are not as durable and stable as the chromium masks, they have proved adequate for the rapid prototyping of microfluidic devices where nano-scale resolution is not an issue. If the masks are printed with a high resolution, 2400 dpi printer, dot sizes of 10 μm can be achieved which highlight their suitability for most microfluidics applications.