1. Introduction
Dehydration reactions are common in chemical industry, and similarly the utilization of carbon dioxide often starts with its chemical reduction with hydrogen and the formation of steam. In fact, the effective handling of steam is one of the biggest bottlenecks for CO2 utilization in industry [1], [2]. Whereas H2O is the main byproduct of many equilibrium limited reactions, such as the water-gas shift reaction and dimethyl ether synthesis, its in situ removal will result in significant conversion enhancements and thus process intensification. This is based on Le Chatelier’s principle, according to which the reactant conversion to products in an equilibrium limited reaction is increased by selectively removing reaction products. In chemical process intensification this concept is used in reactive separations, where reaction and separation take place in a single process step [3]. Another benefit of in situ steam removal involves the mitigation of catalyst deactivation. Thermally induced deactivation (such as sintering or phase transformation at high temperatures) is generally accelerated by H2O [4]. For reactions in which H2O is a main byproduct, such as Fischer-Tropsch synthesis, catalyst deactivation by the formed H2O could be detrimental. On the catalyst level, tuning of catalyst support, promoters and crystallite size has been applied to the increase H2O resistance and extend catalyst lifetime. Additionally, on a reactor and process level, in situ removal of steam would benefit catalyst stability, enhancing catalyst lifetime and process efficiency in these cases as well. While the advantages of process intensification through steam separation enhanced reactions has become apparent from several experimental and theoretical studies, a critical review is currently lacking.
Several possibilities exist to enhance process efficiency by steam separation. Staged ex situ separation, using for example interstage cooling and condensation, could be applied when the conversion per reactor stage is reasonably high. Otherwise, the required large number of sequential reaction and separation steps to achieve low steam levels makes this a less efficient approach [5], [6], or entails the use of a large recycle stream. In situ steam separation by means of a reactive separation method is often required to achieve efficient process intensification.
Although reactive distillation (RD) is one of the best-known examples of integrated reaction and separation and therefore a widespread implemented method [7], [8], its use is limited to systems with a product in the liquid phase. For gas phase systems, membrane (reactive vapor permeation) and (reactive) adsorption technologies are the most important separation methods that could be implemented.
Also membrane technology has been extensively studied and has found various commercial applications, for example in hydrogen separation and filtration applications. Many different types of membranes exist, which are applicable to gas and/or liquid processes. Key factors for application of membrane separationprocesses or membrane reactors, irrespective of the specific process or reactor, are permeability, selectivity or separation factor and material stability [9]. Over the past decades dehydration of organic streams using membranes gained attention with the first steam selective membranes being reported [10]. This development has made membranes available for in situ H2O removal during steam producing reactions, i.e. reactive vapor permeation.
Adsorption technology is widely used in pressure swing adsorption (PSA) applications, where high purity requirements have to be reached. While the concept of reactive adsorption had long been known [11], the use of an adsorbent and catalyst mixture in a reactor and its periodic operation have been studied in the open literature since the 1980s [12], [13], [14], [15]. The most common application of this concept is the conversion enhancement in a reversible reaction to overcome equilibrium limitations. However, selectivity control could also be obtained by the selective removal of a byproduct. The use of a solid adsorbent requires periodic regeneration to regain the adsorptive capacity of the system. Several reactor and regeneration concepts exist for various adsorption technologies. Typically, a fixed-bed reactor configuration is combined with regeneration cycles. The method of regeneration can be divided into pressure swing, temperature swing, concentration swing, reactive regeneration, displacement regeneration or a combination of these operations [16], [17], [18]. In fact, in adsorptive reactors the regeneration process is often the rate determining step and the regeneration determines the cycle times and the required equipment, and therefore the efficiency and feasibility of the process. However, the regeneration procedure is determined by the requirements and the possibilities of the reactive separation system. Carvill et al. (1996) were the first to experimentally investigate the reactive adsorption of steam in the reverse water-gas shift reaction [19]. They showed the potential of reactive adsorption for the in situ removal of steam during a chemical reaction.
Thus, over the past decades the relevance of reactive separation has been established, as many authors have investigated the possibility of reactive steam separation for their studied processes by theoretical and experimental means. At this point in time, lessons can be drawn about the strategies for developing steam separation enhanced reactions, combining insights from experimental and modelling work in the literature. As the interest in the development of steam separation enhanced reactions is strongly increasing with the development of CO2 utilization processes, these lessons may serve as a guidance for future developments in this field.
In this paper the possibilities of in situ steam separation from dehydration reactions are reviewed. The advances in reactive steam separation by membrane and adsorption technologies are highlighted and the potential of both reactive separation methods is discussed, based on process requirements. Whereas the processes relevant in this field are operated at higher temperatures, a general temperature window starting from 200 °C up to maximally 400 °C is considered. Finally, critical aspects for future development and optimization of reactive steam separation technologies are identified.
2. Reactive separation of steam
2.1. General considerations
The conversion of equilibrium limited dehydration reactions can be enhanced due to an equilibrium displacement by selectively separating steam, resulting in lower remaining steam partial pressures. This is illustrated in Fig. 1, where on the left the thermodynamic carbon distribution for direct dimethyl ether (DME) synthesis is depicted and on the right the experimentally obtained results for sorption enhanced DME synthesis are shown [20]. Not only does the DME yield increase from a conventionally limited 9% for CO2 feed, or 55% for CO feed, to more than 80%, also the CO2 content in the product is decreased to less than 1%. This results in an increased single-pass conversion, less demand on downstream separation units, and smaller recycle streams for all CO2 to CO syngas ratios and especially for CO2 feed.
 Fig. 1. Thermodynamic (maximally possible) carbon distribution versus experimentally obtained results for sorption enhanced DME synthesis. Conditions: stoichiometric H2 to COx feed, COx feed is CO2, CO2:CO 2:1 and CO, including 30% inert, 275 °C & 40 bar [20].
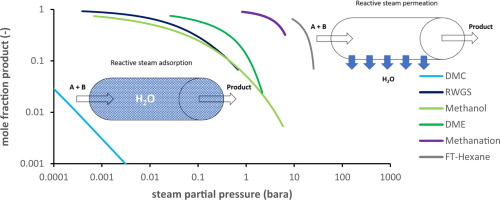
Fig. 1. Thermodynamic (maximally possible) carbon distribution versus experimentally obtained results for sorption enhanced DME synthesis. Conditions: stoichiometric H2 to COx feed, COx feed is CO2, CO2:CO 2:1 and CO, including 30% inert, 275 °C & 40 bar [20].In Fig. 2 the yield as a function of the remaining steam partial pressure is shown for some important reactions studied in literature, clearly indicating the possible increase in equilibrium conversion at lower steam partial pressures for the different reactions. On the one hand, very low steam partial pressures are required to gain a high CO yield in the reverse WGS, or even extremely low partial pressures for the direct synthesis of dimethyl carbonate (DMC). On the other hand, for the Fischer-Tropsch synthesis of hexane a steam partial pressure of 10 bar would already result in significant conversion enhancement. Besides conversion enhancement due to equilibrium displacement, the decrease in the steam partial pressure by in situ separation can also affect the reaction kinetics, reaction selectivity, catalyst deactivation and thus catalyst lifetime. All these positive and/or negative effects are different for each case and must be addressed case specific. For example, in the case of methanation, the primary advantage of employing sorption enhancement is not to increase the (already high) conversion, but to enhance product purity by converting remaining hydrogen [21] or carbon dioxide [22]. Other opportunities are operation at milder reaction conditions to achieve the same conversion and yield as conventional processes, such as in Ref. [19].
 Fig. 2. Molar fraction of product, CO for RWGS, CH3OH for methanol synthesis, DME for direct DME synthesis, CH4 for methanation, hexane for Fischer-Tropsch and DMC for direct DMC synthesis, as a function of the remaining steam partial pressure based on minimization of the Gibbs free energy of reaction. Conditions: stoichiometric H2, CO2 feed for all reactions; RWGS 300 °C, 10 bar; methanol 250 °C, 30 bar; DME 275 °C, 30 bar; methanation 300 °C, 10 bar; FT 250 °C, 30 bar; DMC 200 °C, 30 bar.
Fig. 2. Molar fraction of product, CO for RWGS, CH3OH for methanol synthesis, DME for direct DME synthesis, CH4 for methanation, hexane for Fischer-Tropsch and DMC for direct DMC synthesis, as a function of the remaining steam partial pressure based on minimization of the Gibbs free energy of reaction. Conditions: stoichiometric H2, CO2 feed for all reactions; RWGS 300 °C, 10 bar; methanol 250 °C, 30 bar; DME 275 °C, 30 bar; methanation 300 °C, 10 bar; FT 250 °C, 30 bar; DMC 200 °C, 30 bar.The sections below discuss adsorptive and membrane-based processes, respectively, for steam separation enhanced reaction processes. It is followed by a third section discussing their relative merits.
2.2. Reactive steam adsorption
Steam adsorption enhanced reverse water-gas shift experiments in a bench scale reactor by Carvill et al. were the first to be reported in the open literature [19]. Since then, in situ steam adsorption has been studied for various reactions, including the reverse water-gas shift, the Claus process, the Sabatier process, dimethyl ether (DME) synthesis and dimethyl carbonate (DMC) synthesis. A shortlist of steam adsorption studies is presented in Table 1.
Table 1. Shortlist overview of experimental work on reactive steam adsorption.
| Reaction | Sorbent | Regeneration | Reference |
|---|---|---|---|
| r-WGS | 13X | Pressure swing | Carvill et al. [19] |
| r-WGS | 4A | – | Haije et al. [23] |
| r-WGS | 13X, 4A, SOD | – | Ghodhbene et al. [24] |
| Claus | 3A | Purge & Temperature swing | Agar [25] |
| Claus | 3A | Purge & Temperature swing | Elsner et al. [26], [27] |
| DME | MgSO4 | – | Kim et al. [28] |
| DME | 3A | Pressure swing | Ressler et al. [29] |
| DME | LTA | Temperature & Pressure swing | Boon et al. [5] |
| DME | LTA | Temperature & Pressure swing | van Kampen et al. [20], [30] |
| Methanation | 4A | Purge & Temperature swing | Walspurger et al. [21] |
| Methanation | 3A, 5A | – | Borgschulte et al. [22], [31] |
| Methanation | 5A, 13X | Purge | Delmelle et al. [32], [33] |
| DMC | 3A | – | Choi et al. [34] |
| Glycerol carbonate | 13X | – | George et al. [35] |
Only few authors have conducted studies that combine theory and experiments in a fundamental way, which is crucial for a proper understanding of this type of process as argued below.
Essential for an adsorptive reactor is the capacity of the adsorbent and its affinity for separation of the desired component. However, the capacity of the adsorbent is finite, which makes periodic regeneration inherent to any adsorption process. The periodic regeneration restores the adsorptive capacity and therefore gives an extra degree of freedom to the process, making the method of regeneration another essential aspect for reactive steam adsorption. In this respect, a distinction has to be made between the total capacity and the working capacity of an adsorbent material. The working capacity of the material is its apparent capacity over many consecutive cycles of adsorption and regeneration, whereas the total capacity is a material characteristic given by its isotherm. A large amount of adsorbent material is necessary when its working capacity is low (while a large amount of steam has to be separated). Evidently, a large amount of adsorbent would make the implementation of an adsorptive reactor less feasible. Another factor of interest is the selectivity for the adsorption of steam relative to other components, which determines the loss of reactants and/or products. Non-selective adsorption results in an impure desorption gas stream, which can or has to be separated for economic or environmental reasons. Additional separation units and possible recycle streams make the process more complex, costly and less viable. Besides separation affinity and capacity, the kinetics of separation could also play an important role. Ideally, the rate of steam adsorption matches the rate of steam production. The latter, however, is influenced by the adsorption of steam due the changed kinetics of the desired reaction and/or undesired side-reactions. Moreover, mass transfer limitations between catalytically active sites and adsorption sites could affect the outcome of a reactive adsorption process. In addition to mass transfer, heat transfer is often an important aspect in chemical reactors. Especially for highly exothermic or highly endothermic reactions heat transfer limitations could be problematic and heat management is essential. In addition to the heat of reaction(s), the heat of adsorption has to be managed as well for reactive adsorption processes. This makes the heat management possibly more complex. Consequently, the complexity of operating reactive adsorption processes has led to the proposal of very different contactor types for its implementation. Among others, these contactor types include a moving bed adsorptive reactor, a packed bed adsorptive reactor and a fluidized bedadsorptive reactor. The choice of contactor type influences all other aspects, from heat and mass transfer characteristics to possible regeneration modes. Therefore, one contactor type could be preferred over another depending on specific issues. For instance, a fluidized bed reactor could be beneficial in case of heat transfer limitations. However, a contactor type could also increase the complexity of the process, for example in the case of a moving bed reactor. These aspects of reactive adsorption processes are discussed in more detail below.
2.2.1. Selectivity
For in situ steam removal by solid adsorbents, zeolite type materials are often preferred, as can also be deduced from Table 1. These structured materials have well-defined pore sizes and geometries. Because of their characteristics zeolite materials can have a high affinity and selectivity for the desired adsorbate. Zeolite types 3A and 4A are especially suitable for the removal of steam (~0.2–0.3 nm kinetic diameter [36], [37]) due to their micropores with a diameter of around 0.3–0.4 nm [16], preventing the adsorption of other, larger components that are present during reaction. Conversely, Carvill et al. (1996) used zeolite NaX as water-selective adsorbent at moderately high temperatures for the reverse water-gas shift reaction [19]. Although zeolite NaX typically has a larger pore opening of 0.8 nm, possible co-adsorption of other components is not observed nor discussed. Similarly, zeolite 13X (NaX) is used as solid adsorbent for steam in the formation of larger, cyclic carbonates (e.g. ethylene carbonate, propylene carbonate and glycerol carbonate). Also in this study, enhanced product yields with almost 100% selectivity were obtained [35].
Although co-adsorption is not always observed nor discussed, competitive adsorption of steam and CO2 is well-known for various materials [36], [38]. In line with this, Walspurger et al. (2014) show competitive adsorption of CO2 on zeolite 4A adsorbent. Although the adsorption capacity of CO2 itself seems very low, the capacity for steam adsorption decreases significantly in the presence of CO2 [21]. Recently, Delmelle et al. (2018) gave evidence of a decrease in water diffusion kinetics during adsorbent regeneration due to pore blocking by reaction intermediates and products [32]. Moreover, Vaporciyan and Kadlec (1987, 1989) even use the slightly larger pore zeolite 5A for the separation of CO2 [14], [15]. For zeolites 5A and 4A, authors also showed co-adsorption of other components, such as carbon monoxide, methanol and DME [36], [39], [40]. For zeolite type 3A adsorbent, co-adsorption is less well-known. Its smaller pore size (0.3 nm) physically restricts slightly larger molecules to adsorb, which is why zeolite type 3A is used for drying both polar gases and reactive gases [37]. However, other authors have shown that the presence of methanol influences steam adsorption, even for zeolite type 3A [41], and that DMC can also adsorb on 3A, although at high pressures (20 MPa) [42].
2.2.2. Capacity
Physical adsorption is an exothermic process, which makes that the capacity of all adsorbents decreases with an increase in temperature. A physical sorbent is typically characterized by a low adsorption heat, a low activation energy, high adsorption/desorption rates and excellent reversibility [43]. Contrastingly, chemisorption is characterized by the reaction between adsorbate and surface-active sites of the adsorbent. Therefore, chemisorption features high adsorption heat and activation energy compared to physisorption. Typical chemical adsorbents are base-metal oxides. Although steam will adsorb chemically on base metal oxides, at high temperatures and pressures carbonates will likely be formed in the presence of CO2 [44], [45]. Hydrotalcites, a class of double hydroxides with the general formula Mg(1−x)Alx(OH)2(CO3)(x/2)·nH2O, are typically used for CO2 adsorption. However, they show a capacity for steam adsorption as well [38], [46]. With respect to the sorption capacity, it is important to focus on materials which retain sufficient capacity at elevated temperatures. The steam adsorption capacity of the hydrotalcites, as for the base metal oxides, is however limited [46], [47].
In Fig. 3 (adapted from [48]) the water adsorption capacity of three physical adsorbents is plotted against temperature. Zeolites are well-known molecular sieves, and are used at relatively low to moderate temperatures. Possible steam separation enhanced reactions on the other hand are typically operated at higher temperatures (200–400 °C), which requires sufficient adsorption capacity at these temperatures. Although zeolite molecular sieves have an adsorption capacity for water at slightly elevated temperatures (Fig. 3), little information about the adsorption of steam on zeolite materials under higher temperatures is known in the open literature. Elsner et al. (2002) encountered this issue and experimentally determined a Freundlich isotherm for a zeolite type 3A under sorption enhanced Claus conditions [27]. Other authors, focusing on modelling a sorption enhanced reaction process, use a variety of adsorption isotherm descriptions besides Freundlich models, such as Unilan and Langmuir models, derived from material studies [49], [50], [51]. Gabrus et al. (2015) experimentally studied the water adsorption isotherm for zeolite types 3A and 4A, and Mette et al. (2014) similarly performed a study on binderless zeolite type 13X up to 250 °C [52], [53]. However, it was reported that the latter material has reduced hydrothermal stability at temperatures above 200 °C. Although the Langmuir-Freundlich and the dual-site Langmuir-Langmuir models proposed by Gabrus et al. (2015) are fitted for temperatures up to 250 °C, these, and other descriptions have been determined under different conditions than those of the studied sorption enhanced reactions, which means that the models have to be extrapolated to unverified conditions. In contrast, the more experimental studies regarding sorption enhanced reactions do not explicitly consider isotherm models for the used adsorbent material. The focus in these contributions is merely on the enhancement effect of the adsorbent on the reaction, not on a description of the intrinsic adsorption behavior of the material [19], [22], [28], [29], [34].

Fig. 3. Water adsorption capacity versus temperature in equilibrium with 13.33 mbar water vapor partial pressure for molecular sieves (zeolite 5A), silica gel, and activated alumina (striped lines). Dotted lines show the effect of the 2 wt% of residual water at the start of the adsorption
(adapted from [48]).2.2.3. Regeneration
Besides the adsorption capacity at high temperature, the adsorbent material should also have good adsorption kinetics under the sorption enhanced reaction conditions [24]. Preferably the rates of adsorption and production of steam match. However, especially the desorption kinetics of the adsorbent material are of interest. The desorption rate is often the time-limiting step in a sorption enhanced reaction process, determining the length of the regeneration. The most common regeneration procedures are pressure swing, temperature swing or a combination of both operations, possibly with the use of a purge gas. In theoretical evaluations often pressure swing regeneration is considered [25], [49], [50], because its fast response and therefore short regeneration time is preferred over the slower temperature swing. However, in practical applications of zeolite molecular sieves, the drying beds at moderate temperatures are often regenerated by increasing the temperature [36], [37]. For the use of a purge gas, a suitable stream must be available in the process and its partial back mixing must not cause complications.
In the study by Carvill et al. (1996) pressure swing regeneration is shown to be suitable for periodic regeneration of the zeolite NaX adsorbent [19]. Using product to repressurize the reactor they could achieve a high purity product, whereas reactant repressurization led to sorption enhanced conversions without high purity product. The product CO concentrations were initially low due to the displacement of the pressurization gas from the reactor and only reached a maximum of 80% before reaction equilibrium values were obtained. Although the benefits of sorption enhancement are demonstrated experimentally for the reverse WGS reaction, further optimization of the adsorption and regeneration process is required to roll out the sorption enhanced reverse water-gas shift process to achieve high conversion of CO2 to CO, with high purity. To optimize the sorption enhanced reverse WGS in a solar fuel process Haije et al. (2011) suggest using the heat released from Fischer-Tropsch synthesis, downstream of the rWGS unit, to regenerate the adsorbent by temperature swing regeneration [23].
In the sorption enhanced methanation process temperature swing regeneration with the use of a purge gas has been applied in cyclic experiments, as reported by Walspurger et al. [21]. The regeneration temperature, ranging from 350 to 450 °C, had no significant effect on the working capacity of the used zeolite material, which could be due to either the extensive regeneration time or the excess of purge gas used. Delmelle et al. (2016) observed a significant improvement in regeneration for a hybrid Ni/13X adsorbent using air rather than hydrogen as purge gas in their sorption enhanced methanation process [33]. The purge gas did not affect the regeneration of hybrid Ni/5A, suggesting that the larger pores of 13X allow for faster transport and better regeneration. Whereas the methanation experiments have been performed at atmospheric pressure, the choice of regeneration method and appropriate conditions, however, is critical for the feasibility of the reactor concept. Design and optimization of the regeneration step will be key in optimizing the energy requirement and the associated operational costs.
In successive studies on the Claus process, the regeneration was analyzed in more detail [26], [27]. Based on the thermal inertia of the fixed-bed, the study concludes that regeneration by temperature swing is not feasible. This makes pressure swing regeneration the most viable option, which is often preferred over TSA in the design of cyclic processes. Abufares et al. (2007) evaluate an optimization model for vacuum swing regeneration for the same Claus process [54]. They show that a high performance adsorptive reaction process is possible by means of optimized vacuum swing regeneration.
In addition to previous studies, van Kampen et al. (2017) also explicitly looked into the effect of the regeneration conditions on the sorption enhanced DME process [20], [30]. They have shown that the used zeolite adsorbents can be readily regenerated by pressure swing regeneration. Increasing the temperature during regeneration (temperature swing) improves the extent of regeneration even further by increasing the working capacity of the adsorbent.
2.2.4. Catalyst activity, reaction kinetics, and mass transfer
Studies have clearly indicated that the adsorbent regeneration not only influences the adsorptive capacity of the system, but that periodic exposure to regeneration conditions may also affect the catalyst performance. In a study of the regeneration conditions in sorption enhanced DME synthesis, van Kampen et al. [20] have shown that periodic exposure to temperature swing conditions of 400 °C not only improves the performance of the adsorbent, which can be judged from the extended period before breakthrough, but also improves the activity of the Cu/ZnO/Al2O3 catalyst, which can be judged from the equilibrium conversion after breakthrough and stabilization (see Fig. 4, Fig. 5).
 Fig. 4. Typical breakthrough profile for CZA catalyst + LTA adsorbent system. Conditions: stoichiometric H2, CO, CO2 feed for all reactions; CO2:CO = 1:2; 275 °C, 25 bar(a); 300 °C regeneration [20].
Fig. 4. Typical breakthrough profile for CZA catalyst + LTA adsorbent system. Conditions: stoichiometric H2, CO, CO2 feed for all reactions; CO2:CO = 1:2; 275 °C, 25 bar(a); 300 °C regeneration [20]. Fig. 5. Typical breakthrough profile for CZA catalyst + LTA adsorbent system. Conditions: stoichiometric H2, CO, CO2 feed for all reactions; CO2:CO = 1:2; 275 °C, 25 bar(a); 400 °C regeneration [20].
Fig. 5. Typical breakthrough profile for CZA catalyst + LTA adsorbent system. Conditions: stoichiometric H2, CO, CO2 feed for all reactions; CO2:CO = 1:2; 275 °C, 25 bar(a); 400 °C regeneration [20].Regarding the effect on catalyst activity and reaction kinetics, Reßler et al. (2005), employing adsorbent zeolite 3A in sorption enhanced DME formation, posit that the removal of steam has a conflicting role. Methanol and DME producing reactions are enhanced, but the opposite holds for the water-gas shift reaction [29]. It is widely known that a low content of CO2 enhances the reaction kinetics for methanol synthesis over CZA catalysts, although CO2 limits the reaction equilibrium [55]. Whereas the extent of regeneration in sorption enhanced DME synthesis influences the amount of CO2 present, it also directly influences the reaction kinetics. This gives the regeneration of this reactive adsorption system extra importance, although it is an important parameter in adsorptive reactors in general.
Although sorption enhanced methanation is shown by Walspurger et al. (2014) for a physical mixture of a methanation catalyst and a zeolite adsorbent [21], there are indications that a hybrid catalyst adsorbent particle could perform better than a physical mixture [22], [31]. It is clear that the effective transport of species between, and therefore the proximity of catalytically active sites and adsorbent sites can have an important role in sorption enhanced processes. In addition, this also suggests that the major mass transport resistance comprises the transport from the catalyst, via the bulk phase, to the sorbent. The relatively low space velocities for many adsorption processes increases the likelihood of mass transfer limitations. However, recent developments in rapid PSA cycling seem promising for improving these aspects.
As mentioned previously, by modification of the reaction conditions the conversion of the desired reaction can be enhanced, but undesired parallel or consecutive reactions may be enhanced as well. In the Claus process this aspect of reactive adsorption appears. To a small extent, the formation of undesirable carbonyl sulfide (COS) was observed. In the conventional Claus process COS is hydrolyzed by the steam present in the reactor. Whereas steam is selectively removed in the adsorptive reactor concept, this hydrolyzation is suppressed as well and even more COS could be formed compared to the conventional process [27]. This example of COS formation clearly illustrates the possible enhancement of undesired side-reactions due to inherent concentration or temperature profiles in reactive adsorption processes.
2.2.5. Heat management
Heat management is very important for chemical reactions, especially in the case of high exothermicity or endothermicity, and thus also for reactive adsorption processes. The adsorption of steam is an exothermic process, which is therefore favored at low temperatures (and high pressures). The adsorbent material requires a high affinity for steam to obtain sufficient working capacity and subsequent sorption enhancement at higher reaction temperatures (between 200 and 400 °C). This was shown for the zeolite type adsorbents used by various authors (Table 1).
For exothermic reactions, such as the methanation reaction, the Claus reaction and (direct) DME synthesis, the adiabatic temperature rise could be very high (up to 500 °C for methanation). Besides the influence this already would have on the conventional reactions, these temperature profiles affect the adsorption capacity of steam, the kinetics and the concentration profiles in adsorptive reactions. For this reason, Walspurger et al. (2014) considered an adsorptive reactor as a third reactor in a (conventional) series of three adiabatic reactors, in which the main part of the reaction heat is mitigated to the first and second reactor. In this way they motivate the use of an adiabatic reactor instead of a more complex and costly isothermal reactor [21]. Elsner et al. (2003), on the other hand, claim that the thermal inertia of the fixed bed adsorptive reactor avoids interference of the reaction heat with the adsorption process [27], which would make adiabatic operation feasible. In addition, for a fixed bed reactor in chemical looping combustion it is shown that as long as the velocity of the reaction front is larger than that of the heat front, the maximum temperature increase is only effected by the gas and solid properties [56], [57]. Also other authors assessed the possibilities of adiabatic and non-adiabatic reactor operation. Sorption enhanced reverse WGS couples the endothermic rWGS reaction with the exothermic steam adsorption, possibly minimizing external heat input required for rWGS [58]. In a fixed bed reactor Parra et al. (2017) show that in the optimal configuration the temperature is close to the set maximum temperature of 260 °C. Although a higher temperature limits the adsorption capacity, the reaction kinetics are faster. For the adiabatic case this optimization towards the highest allowable temperature means that the feed temperature has to be low to not exceed 260 °C inside the reactor. In the non-adiabatic case a profile of the wall temperature allows for the highest productivity. Near the reactor inlet a lower wall temperature results in more adsorption due to the higher allowable heat of adsorption. Throughout the reactor the rates of reaction and adsorption converge, allowing operation at higher (wall) temperatures [58]. This shows the advantage of non-adiabatic operation, the temperature can be tuned to favor the dominant phenomenon, reaction or adsorption. Contrastingly, for adiabatic operation the temperature is determined by (the exo- or endothermicity of) the dominant phenomenon, which is also shown for sorption enhanced reverse WGS in a moving bed reactor [59].
2.2.6. Contactor type and reactor configuration
In the literature focusing on reactive steam adsorption, a packed bed adsorptive reactor is the most commonly selected reactor configuration [19], [20], [21], [27]. This reactor configuration is relatively easy to implement compared to a less mature technology, such as a fluidized bed adsorptive reactor. By optimizing the reactor operation, the ideal performance of a moving bed reactor can be achieved. Parra et al. (2017, 2018) showed that a moving bed adsorptive reactor could be more beneficial than a packed bed adsorptive reactor for the reverse WGS reaction with an order of magnitude difference in space time yield [58], [59]. Similarly, Santos et al. (2015) proved the potential of a moving bed chromatograph reactor for the separation of water and DMC, in its direct synthesis [60]. Although other contactor types are less apparent in research on reactive steam adsorption, they could be beneficial for specific steam sorption enhanced reaction processes. In the previous sections the importance of mass transfer and heat management is discussed. If transfer limitations are a serious issue for a process, fluidized bed technology would be a good candidate for its great mass and heat transfer properties. For these reasons Bayat et al. have suggested several configurations for sorption enhanced methanol synthesis and sorption enhanced Fischer-Tropsch synthesis [51], [61], including a dual (moving) bed reactor and a fluidized bed reactor, thermally coupled to a fixed bed reactor.
2.3. Reactive steam permeation (membrane steam separation)
The second reactive steam separation method is reactive steam permeation or reactive membrane separation. Few studies have been published on steam separation enhanced reactions, including DME and DMC synthesis [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], however the Fischer-Tropsch (FT) reaction has received the most attention [73], [74], [75], [76], [77], [78]. A shortlist is given in Table 2.
Table 2. Shortlist overview of experimental and modelling literature on reactive steam permeation (and pervaporation). Membrane reactors (MR), packed bed membrane reactors (PBMR), catalytic membrane reactors (CMR) and simulated moving bed membrane reactors (PermSMBR) are listed.