Highlights
-
•
A comprehensive review on sodium carbonation pathways for CO2 emission reduction is presented.
-
•
The chemistry, key parameters and simulation studies in NaHCO3 synthesis for carbon capture and utilization process is reviewed.
-
•
The advanced modification technologies that enhance the process efficiency and economic viability are discussed.
Abstract
Carbon capture and utilization (CCU) stands as a pioneering solution to counter greenhouse gas emissions linked to fossil fuel consumption. Research in the utilization of CO2 with sodium-rich sources, particularly industrial waste, for the synthesis of sodium bicarbonate (NaHCO3) has made significant progress. Despite the potential benefits, challenges such as sluggish reaction kinetics, solvent evaporation, product purity concerns, and the generation of secondary waste have hindered the widespread commercial adoption of this process. This comprehensive review delves into the various sodium carbonation pathways used in the conversion of CO2, focusing on advanced modification technologies that offer viable solutions to enhance the efficiency and economic viability of the process. The study meticulously explores the different methods employed for sodium bicarbonate synthesis, encompassing soda ash carbonation, Solvay process, ammonia sulphate, sodium hydroxide, and the electrochemical conversion of CO2. The essential chemical reactions, crucial precipitation parameters, and simulation and modelling endeavors aimed at upscale implementation of the carbonation associated with each carbonation method are thoroughly discussed. The incorporation of process and mass transfer intensification approaches can enhance the carbonation process, unlocking the potential of sodium carbonation pathways for a sustainable CCU process. Over all, the study aims to shed light on the existing constraints and pave the way for future innovation and effective implementation strategies in the field of carbon capture and utilization.
Keywords
Carbon dioxide utilization
Sodium bicarbonate
Carbon capture efficiency
Industrial saline waste
crystallization
1. Introduction
The Industrial Revolution's increased energy consumption from fossil fuels has generated significant carbon dioxide (CO2) emissions, contributing to climate change and significant environmental impacts [1]. Carbon capture and storage (CCS) systems have been developed as a means to reduce CO2 emissions. CCS involves separating carbon dioxide from emission sources, compressing it, and transporting it to a suitable location for permanent storage 2, 3. The post-combustion CCS technique has undergone thorough research and has been commercialized in energy plants and industrial sectors 4, 5. However, there are some drawbacks associated with energy consumption 3, 6, 7, and many countries lack the necessary storage spaces [8].
CCS projects are transitioning towards carbon capture and utilization (CCU) [9]in order to address the challenges associated with CCS and to guarantee the safety, economic viability, and sustainability of CO2 removal technologies [10]. CCU technologies have been devised to convert CO2 into compounds with substantial added value without the need for energy-intensive desorption procedures 11, 12. While the ability to reuse captured CO2 may mitigate a portion of the overall expense associated with carbon capture and storage, most schemes are plagued by two persistent problems. A primary factor is the significant stable thermodynamic property of CO2, necessitating a substantial input of energy to transform molecular CO2 into a suitable end product 13, 14. Another significant issue is the requirement to provide hydrogen to convert CO2to alkanes, such as methanol and methane [15].
CO2 mineralization circumvents the disadvantages associated with the majority of CCU methods by transforming CO2 to bicarbonates and/or carbonates, hence shifting CO2 to a lower energy state [16]. This could be accomplished by introducing it into an alkaline solution or by allowing it to adsorb onto a solid [17]. The products may be sold in their original form [18] or subjected to heating to produce pure CO2 gas [19]. Nevertheless, the former option is more desirable due to its avoidance of extra energy input and its exclusive production of molecular CO2, the disadvantages of which have previously been deliberated. It has been demonstrated that mineral carbonation is the most promising option for commercializing large-scale carbon capture plants, primarily because of its thermodynamic advantages and economic feasibility 20, 21. Experimental studies have shown that mineralization can be accomplished to produce valuable compounds, such as sodium bicarbonate (NaHCO3), calcium carbonate (CaCO3), and others 22, 23. These technologies have been successfully utilized in industrial environments in recent years 24, 25. On the other hand, the utilization of industrial saline wastes for mineralization has emerged as a compelling approach to directly and indirectly reduce CO2 emissions from industrial and power plant activities. Although natural ores have a substantial capability to collect CO2, mineralization utilizing alkaline solid wastes offers additional advantages, such as lower cost of raw materials and proximity to the CO2 source [26].
The CO2 mineralization, leading to the formation of sodium bicarbonate, is experiencing a surge in global demand owing to its multifaceted applications across various industries. Research has been conducted to propose more efficient methods for producing sodium bicarbonate that offer improved conversion rates, the ability to use available ingredients, and economic viability, intended to reduce CO2 emissions 18, 27, 28. Sodium bicarbonate is used as a growing ingredient in the production of food industries, chemical industries, gold processing, the production of wool and silk dyes, leather goods, etc. Additionally, it serves as a primary component in fire extinguishers and as a suppressant of explosions in the handling of combustion flue gas 29, 30, 31, 32. The projected value of the sodium bicarbonate market in 2018 was around USD 1.2 billion. Furthermore, there is an escalating need that is projected to increase by around 2-5% every year until 2025 [33].
The recent review papers 26, 34, 35, 36, 37, 38, 39, 40, 41, 42 that cover the synthesis of metal carbonates/bicarbonates in carbon capture and storage technologies or carbon dioxide utilization are listed in Table 1. They are useful references to gain basic knowledge about the individual topics. The exploration of mineral carbonation for the production of CaCO3 and MgCO3 has gained significant attention, as evidenced by the emergence of several comprehensive review papers evaluating the development prospects in detail. However, the focus on the utilization of CO2 for the production of NaHCO3 and the gaps have been relatively overlooked in recent review literature. Studies assessing the CO2balance have unequivocally demonstrated that NaHCO3 precipitation exhibits remarkably higher CO2 uptake compared to the precipitation of CaCO3 and MgCO3 [12]. Notwithstanding its potential, the carbonation process encounters challenges associated with slow reaction rates, necessitating larger carbonation column volumes, solvent vaporization, product purity, and secondary waste pollution.
| Topic | Goal of the review | Year | Ref. |
|---|---|---|---|
| Production of sodium bicarbonate from CO2reuse processes: a brief review | To present methods of obtaining sodium bicarbonate by means of crystallization reactions processes reusing carbon dioxide. | 2019 | [34] |
| Recent advances in carbon dioxide utilization | To examine the latest developments in carbon dioxide capture, utilization, conversion, and sequestration from a multi-scale perspective. | 2020 | [26] |
| Simultaneous treatment of reject brine and capture of carbon dioxide: A comprehensive review | To investigate the combined treatment of CO2 and utilization of reject brine to achieve high performance. | 2020 | [35] |
| A critical review to bridge the gaps between carbon capture, storage and use of CaCO3 | To investigate the gaps between carbon capture technologies and carbonation of calcium rich particles for the production of modified CaCO3 for its implementation at large scale. | 2020 | [36] |
| Utilization of gaseous carbon dioxide and industrial Ca-rich waste for calcium carbonate precipitation: A review | To present the calcium-rich waste for the carbonation routes to produce CaCO3, while also discussing the possible limitations and implementation problems of the proposed technologies. | 2020 | [37] |
| Review of carbon capture absorbents for CO2utilization | To discusses the importance of evaluating CO2 utilization routes and the incorporation of advanced technologies and business models for accelerating the development of carbon capture technologies. | 2022 | [38] |
| A review on chemical precipitation in carbon capture, utilization and storage | To examine fundamentals of precipitation and dissolution of calcium and magnesium from silicates or industrial waste to precipitate CO2. | 2022 | [39] |
| Review of contemporary research on inorganic CO2utilization via CO2conversion into metal carbonate-based materials | To provide information on the basic principles of carbon dioxide conversion into metal carbonate-based | 2022 | [40] |
| A review on the selection criteria for slow and medium kinetic solvents used in CO2 absorption for natural gas purification | To assess the critical selection criteria for CO2 absorption solvents emphasizing the importance of absorption rate enhancement research for improved solvent performance in industrial applications. | 2022 | [41] |
| Mineral carbonation using seawater for CO2sequestration and utilization: A review | To examine the latest developments and improve the efficiency of precipitating magnesium and calcium in carbon dioxide mineralization using seawater. | 2023 | [42] |
Therefore, the primary objective of this review paper is to delve into a thorough exploration and analysis of various sodium carbonation technologies utilized for the production of value-added sodium bicarbonate. This investigation places a specific emphasis on the modification techniques employed to enhance the carbonation process, ultimately leading to a higher CO2 conversion rate. This comprehensive review encompasses an in-depth discussion of the sodium carbonation pathways, including the utilization of sodium-rich industrial wastes and their influence on carbon capture efficiency. Furthermore, it scrutinizes the crystallization process of NaHCO3 for each carbonation route while investigating the impact of various parameters. Moreover, this review assimilates studies conducted on simulation and modeling, focusing on scaling up the carbonation process. In conclusion, this paper summarizes the current state of the art, paving the way for future investigations and innovations aimed at achieving net-zero industrial waste emission. This conclusive discussion provides useful insights into an economically viable and sustainable approach for CO2 utilization and NaHCO3 production, thereby offering a foundation for further advancements in the field.
2. Sodium carbonation routes
Strategically used as a low-cost raw material, sodium plays a pivotal role in CO2utilization within industrial processes. Its abundance and accessibility position it as an attractive option for sustainable manufacturing practices that prioritize environmental stewardship. Utilizing naturally occurring minerals and industrial saline waste rich in sodium for carbon capture strategies provides valuable materials such as the precipitation of sodium bicarbonate (NaHCO3), aligning with circular economy principles and resource optimization. The main sodium carbonation pathways for the precipitation of NaHCO3 in the CCU process are soda ash carbonation, Solvay process, ammonia sodium sulfate carbonation, NaOH carbonation, and electrochemical conversion of CO2. The following part provides a concise overview of the utilization of CO2 as a reactant in the process of producing sodium bicarbonate. This aims to improve understanding of the distinctive performance attributes associated with each route and prospective for future studies.
2.1. Soda ash carbonation
Soda ash carbonation is a commonly used method in industrial settings to make sodium bicarbonate (NaHCO3) using Na2CO3 obtained from trona. Natural trona contains approximately 46% Na2CO3 and 35% NaHCO3, is abundantly found in nature, and is extensively spread worldwide [43]. The techniques used in trona mining processes include the monohydrate and the sesquicarbonate phases [44]. The first stage of the process is the monohydrate process, which entails smashing the trona minerals, removing water and CO2 using a calcination method to generate sodium carbonate, and then reacting the sodium carbonate with CO2 to provide the end product, sodium bicarbonate. The reaction that yields Na2CO3 as a result of thermal decomposition of the trona is given by [45]:(1)
Theoretically, the temperature range of 57 °C to 100 °C is considered suitable for the conversion of trona into sodium bicarbonate [46]. The overall soda ash dissolution and carbonation reactions are as follows [34]:(2)
The forward reaction Eq. (6) is quite exothermic 47, 48, and crystallization reaction rate is affected by temperature, sodium carbonate concentration, and CO2 flowrate 49, 50. The presence of CO2 and vaporized water is also crucial for the conversion process. The rate of carbonation accelerates in direct proportion to the concentration of CO2 in the gas phase and the relative humidity level which ranges from 50 to 70%. Nevertheless, both excessively high and excessively low levels of humidity impede the pace at which carbonation occurs 51, 52. Equilibrium between carbonate and bicarbonate ions in the aqueous solution also determines the stable solid phase in a saturated solution and the corresponding solubility concentrations of carbonate and bicarbonate. This equilibrium is strongly affected by the CO2 partial pressure. At temperature < 80 °C (equilibrium constant (Ks) NaHCO3 < 1) and ambient CO2 partial pressure, solid bicarbonate (nahcolite) is the thermodynamically stable solid.
Cho et al. [43] found a satisfactory results for the conversion trona to NaHCO3 at higher temperature like 42 °C, 50 °C, and 70 °C. This would be related to the structural variation of trona particles at the lower temperature. A trona particle is initially nonporous and then it begins to crack so that fissures appear on the surface. This structural variation creates more surface area for the reaction with CO2 and water vapor. However, at the lower temperature, a trona particle takes longer time to change its structure. In other words, the fissures on the surface of the particles are not fully developed at the lower temperature during the beginning of the reaction. As a consequence, the level of the conversion of trona at the lower temperature is low during the beginning of the reaction and the time to approach the complete conversion is shorter as temperature increases. Table 2 outlines the sodium bicarbonate precipitation technologies using soda ash carbonation process.
| Na+Source | Reactants | Parameters | Reactor | Result/ main product& CO2 capture efficiency | Ref. |
|---|---|---|---|---|---|
| Na2CO3 |
0-30% Na2CO3 13.5% CO2 |
Temp=45–70°C Liquid/gas ratio= 2.1–5.5 |
Packed column |
NaHCO3 90 % |
[53] |
| Trona |
Trona (mineral) 10–15% CO2 |
Temp = 60–70°C | Carbonation tower |
NaHCO3 90 % |
[54] |
| Na2CO3 | Liquid mixture of Na2CO3 (2.72%), NaHCO3 (2.99%), and H2O (94.28) |
Temp=50°C Gas flow rate = 200 Nm3/h |
Industrial bubble column |
NaHCO3 25–30 voltric % of CO2was absorbed. |
[55] |
| Hydrated sodium carbonate |
Hydrated Na2CO3 99% CO2 |
Temp=30–50°C | Glass container |
NaHCO3, CO2 capture capacity 282 mg/ g of solution. (90% saturation uptake within 16 m) |
[56] |
| Na2CO3from Solvay process |
Na2CO3 Solution 12–15% CO2 |
Temp= 38−45 °C pH= 8.5−9.0 |
Transparent acrylic material carbonation tower |
NaHCO3(purity >99%) 0.33 ton of CO2 utilized to produce 1 ton of NaHCO3 |
[27] |
| Trona | Trona (mineral), 12% CO2 | Temp=10–70°C | Carbonation tower |
NaHCO3, CO2/purity 99.90% CO2 Capture efficiency=90 % |
[57] |
| Frother-enhanced Na2CO3 |
Na2CO3 solution 16% CO2 |
Temp=25−60 °C Gas flow rate=21LPM Liquid flow rate= 3–10L/min |
Packed bed with Polypropylene pall rings |
The absorption efficiency increased from 55.6% to 99.9% |
[58] |
2.2. Solvay process
The Solvay process (also called ammonia-soda process) is another process that was originally designed for the production of sodium carbonate and is currently being used for the production of sodium bicarbonate in CO2 utilization process. It involves the reaction of a concentrated sodium chloride solution with NH3 and CO2 to produce soluble ammonium bicarbonate. This ammonium bicarbonate then reacts with NaCl to yield soluble ammonium chloride (NH4Cl) and a precipitate of NaHCO3. The aqueous solution of NH4Cl obtained may undergo a reaction with calcium hydroxide (Ca(OH)2) in order to recover and reuse the NH3[59].
In the conventional Solvay method, the chemical reactions involving CO2 and ammoniated brine can be classified as primary and secondary. During the first phase, after the water dissociation in Eq. (7), CO2 undergoes a chemical interaction with ammonia (NH3) to produce carbamic acid. An additional reaction between carbamic acid and NH3 produces ammonium carbamate [60]. The primary phase reaction is expressed as follows:(7)
Therefore, the overall reaction is given as:(12)
The primary reaction is rapid compared with the hydrolysis of the carbamate in the bulk of the solution. The secondary reaction entails the slow hydrolysis of carbamate to bicarbonate.(13)
The bicarbonate ions precipitate as sodium bicarbonate.(14)
The aforementioned phases include the process of mass transfer accompanied by a chemical reaction. The total yield of the reaction is influenced by the concentrations of NH3 and CO2. The absorption of CO2 into strongly carbonated ammoniated brine solution is characterized by a relatively low rate of absorption [61]. As described in Eq. (12), the primary reaction rapidly consumes CO2, and at a high carbonation time, the system is under diffusion control. Eq. (13) states that as CO2 absorption rate increases, both the yield and the secondary reaction increase. The rate of reaction at any given moment, which is determined by the diffusion of the chemical reaction, can be expressed as:(15)
Sodium bicarbonate (NaHCO3) is an essential intermediate compound in the Solvay carbonation process, in which its solubility is a key factor for the process to be successful. In order to maximize conversion, it is important to minimize the solubility of NaHCO3 by optimizing the variables that might affect or decrease its solubility. The main parameters that affect the carbonation process include pH, temperature, gas flow rate, and ammonia concentration.
The pH level is the key factor for assessing the dominant carbon form in the ammoniated brine carbonation process. This is important to maximize both the CO2 absorption capacity and the bicarbonate proportion. Fig. 1 illustrates the pH level impact on the relative quantity of bicarbonate ion (
 Fig. 1
Fig. 1Another important factor is temperature, which has an impact on CO2 capture efficiency, conversion rate, product solubility, and composition of the end products in the formation NaHCO3 using the Solvay process. Increasing the temperature leads to an elevation in the solubility of the product, reducing the CO2 capture efficiency and NaHCO3 formation, resulting in alterations in the composition of the product. Moreover, increasing temperature accelerates the NH3 escape, leading to a reduction in the pH of the ammoniated brine solution and eventually causing a decline in CO2 absorption. On the other hand, at lower temperatures, the separation process becomes less effective due to the slower CO2 reaction rates with aqueous ammonia. The optimum temperature is found to be 22°C for an ammoniated brine solution containing 2.5 M NH3 and 4.3 M NaCl [63]. The rise in temperature from 10 °C to 50 °C results in an almost 50% decline in CO2 removal efficiency and a reduction in Na removal efficiency, dropping from 45.2% to less than 5% in a brine reject brine solution [64]. Increasing gas flow rate with increasing temperature worsened the decline in carbon capture efficiency, which can be attributed to the shortened residence time of the gas [65].
Ammonia concentration had a greater impact required to absorb larger amounts of CO2 66, 67, 68. Ammonia does not participate in the main reaction of the Solvay process, but it does have a crucial function in the intermediate reactions. The presence of ammonia maintains the solution at an alkaline pH, which is essential for facilitating the precipitation of sodium bicarbonate.
2.3. Sodium sulfate carbonation process
The sodium sulfate carbonation route presents a promising strategy for capturing CO2 emissions, with a capacity to capture 1.04 kg CO2/kg of NaHCO3[28]. Technoeconomic analysis demonstrates its cost-effectiveness, favoring the production of baking soda over soda ash [69]. Originally designed as an upgrade to the Solvay process, this method eliminates the need for calcareous material and enables the extraction of gaseous HCl from NaCl. The process itself is complex involving the transfer of heat, mass, and momentum in three phases, and includes intricate reversible chemical reactions and crystal formation. Additionally, the chemical reactions involved in the process are influenced by the compound's solubility in relation to temperature [70], resulting in the formation of ammonium sulfate as an intermediary product. The following equations illustrate the involved reactions [34].(16)
The carbonation stage of the ammoniated sodium sulfate process involves an overall chemical reaction Eq. (24), with ions exhibiting affinities due to the standard negative Gibbs energy [71]. However, a significant limitation arises from the co-precipitation of ammonium sulfate with sodium sulfate in the form of a double salt compound, potentially rendering the method less commercially viable. While simplified batch experiments demonstrated the ability to generate sodium carbonate and purified ammonium sulfate simultaneously [72], the continuous prototype scale revealed undesirable process limitations, particularly the risk of contamination and simultaneous precipitation of undesired compounds [34]. Nevertheless, a technique has been proposed for producing high-quality baking soda and purified ammonium sulfate as a byproduct, suitable for commercial use as a fertilizer 73, 74.
Controlling reaction parameters plays a crucial role in ensuring the successful crystallization of pure NaHCO3. Among these parameters, the flow of NH3 is particularly significant in the production process. Increasing the flow rate of a liquid, would result in a tremendous amount of liquid being dispersed over the packing surface, which would cause an increase in the interfacial area of the two phases. This leads to a higher liquid mass transfer that is strongly related to carbon capture efficiency in a liquid-phase controlled mass transfer 75, 76. Research findings suggest that higher NH3 flow rates are favorable for CO2capture, with excessive flow rates showing minimal effect on process performance. On the other hand, the molar flow rate of mirabilite is another important parameter that affects the carbon capture efficiency and production of NaHCO3. The gradual increment of the molar flow rate of mirabilite from 5 kmol/h to 8 kmol/h, increased the CO2 capture efficiency from 49.21% to 59.24% [77]. Then it gradually flattened, because the content of feed water remained unchanged, and the mirabilite solution became saturated. The results show that the use of saturated mirabilite is beneficial for the improvement of CO2capture efficiency.
Another essential parameter in the production of sodium bicarbonate is crystallizing temperature. Studies showed that too a high crystallizing temperature is not conducive to CO2 capture and NaHCO3 crystallization, and the optimal reaction temperature is at 35.5 ◦C [77]. It has been observed that lower absorption temperatures lead to faster absorption rates under experimental conditions. This phenomenon can be attributed to the inverse relationship between temperature and the solubility coefficient of Henry’s law, as well as the absorption driving force. The rise in the quasi-steady-state partial pressure of CO2 with increasing absorption temperature contributes to this effect [78].
2.4. Sodium Hydroxide (NaOH)
Many studies have been conducted to reduce CO2 emissions by employing NaOH 76, 79, 80. The CO2 absorption capacity of NaOH is significantly greater than that of monoethanol amine (MEA) absorbent, in which the estimated absorbing capacity of NaOH is 1.11 tons CO2/ton NaOH while MEA is approximately 0.72 tons CO2/ton MEA [81]. Furthermore, NaOH is readily available and less expensive in comparison to MEA [82]. In recent times, there has been considerable interest in the application of NaOH for the production of NaHCO3, a method that significantly reduces industrial CO2 emissions [83].
In order to analyze the reaction between NaOH and CO2 more thoroughly, the various stages of the absorption mechanism must be considered. In the first place, the aqueous solution of NaOH rapidly and fully ionizes into Na+ and OH−ions because of its high alkalinity.(25)
Injecting gaseous CO2 into a NaOH solution produces an aqueous solution of CO2 [84]. When aqueous CO2 interacts with a hydroxide ion, it produces bicarbonates. At first, there are many hydroxide ions in the solution. As a result, the bicarbonate ions interact with hydroxide to form carbonates as expressed in Eqs. (27), (28). The carbonate ions react with sodium ions in the aqueous solution, resulting in the formation of Na2CO3, Meanwhile, the remaining hydroxide ion concentration is reduced. The loading curve and real-time changes in pH confirm this.(26)
Reaction of Eq. (27) is a second-order reaction, Eqs. (27), (28) are reversible chemical reactions that occur rapidly in high pH ranges [85]. Reaction Eq. (28) is carried out immediately after reaction Eq. (27) [86]. With a continuous input of CO2, the concentration of carbonate ions will be higher while the concentration of hydroxide ion will be reduced, hence, the pH of the solution decreased. According to Le Chatelier's principle, the reverse reactions in Eq. (28) will dominate, thereby increasing the amount of
The overall CO2 absorption reaction with NaOH in an aqueous solution can be described as reaction Eq. (31),(31)
The pH level is an essential indication for evaluating the progression of the reaction in order to determine the predominant form of carbon and maximizing both the CO2 absorption capacity and the bicarbonate proportion. A higher concentration of bicarbonate ions results in a proportional increase in the removal of sodium ions, as stated in Le Chatelier's principle [87].
Yoo et al. [82] investigated a concentration of 1 to 5 wt.% of NaOH solution to capture a gas mixture with 31.5 vol.% CO2 and 68.5 vol.% N2 in a Pyrex reactor. The CO2 absorption process was conducted in a series of sequential reaction steps, with Na2CO3 and NaHCO3 being formed. During the Na2CO3 production range, the reaction rate and capture efficiency exhibited a significant dependence on the concentration of NaOH. However, during the NaHCO3production phase, these parameters remained constant regardless of the NaOH concentration. The absorbed CO2 mass ratio that took part in the trona, NaHCO3, and Na2CO3 production reactions was calculated to be 1:17: 20, respectively. In another study, Leventaki et al. [88] utilized a 3D-printer to develop a specialized reactor for assessing the CO2 absorption capabilities of NaOH solutions within a concentration ranging of 1 to 8 w/w%. When the concentration of NaOH reaches 5 w/w% or greater, solid carbonate and bicarbonate compounds are produced as a result of saturation. They obtained absorption capacities ranging from 9.5 to 78.9 g CO2/L at NaOH concentrations of 1 w/w% and 8 w/w%, respectively.
Apart from that, the effect of temperature on the CO2 capture efficiency of the NaOH system was investigated by Kordylewski et al. [89]. From the result, it was shown that by increasing the absorption temperature from 25 to 61.5◦C, the overall CO2 capture efficiency can be improved by approximately 5–20%. It was also stated that after the formation of Na2CO3 post absorption by NaOH, the carbonate can still capture CO2 to form NaHCO3 but with a relatively low CO2capture efficiency of 4–5%.
2.5. Electrochemical conversion of CO2
The electrochemical conversion of CO2 into valuable compounds is receiving increasing interest due to its versatility, capacity to tackle emissions from dispersed sources (such as the ocean and atmosphere), and its compatibility with an electrified industrial facility 90, 91, 92. The process of electrochemical conversion of carbon dioxide has been successfully shown in a fuel cell, which was achieved by employing a specific catalyst that facilitated the conversion of carbon dioxide into carbonate ions that served as a means of transferring charge species [93]. Recently, a multi-compartment electrodialysis device utilizing an effective combination of anion exchange membranes (AEM) and cation exchange membranes (CEM) has successfully proven the simultaneous removal of carbon dioxide in a mineralized state. The captured CO2 typically contains impurities that need to be removed before further processing. After the captured CO2 is fed in to the electrochemical cell, the CO2 is electrochemically converted to sodium bicarbonate (NaHCO3) in the presence of water (H2O) and a suitable electrolyte at the cathode. The electrolyte can be a solution of sodium salts, such as sodium hydroxide (NaOH) or sodium carbonate (Na2CO3). These sodium salts dissociate in the electrolyte, releasing sodium ions (Na+) that can participate in the electrochemical reaction at the cathode. During the electrochemical reduction of CO2, the sodium ions (Na+) combine with the bicarbonate ions (
The electrochemical reaction of water splitting involves the hydrogen evolution reaction (HER) at the cathode and the oxygen evolution reaction (OER) at the anode which can be presented:(32)
By injecting CO2 into the cathode gas supply, where it reacts with the hydroxide ions produced at the cathode,
The selection of catalysts on the electrode surface, together with the composition and concentration of the electrolyte, plays a crucial role in defining the effectiveness of the process in terms of capturing, transporting, and releasing CO2, as well as the kinetics involved [94].
3. Carbonation using Na+-rich industrial wastes
CCU technology has the potential to markedly decrease carbon emissions and foster sustainable economic prospects. According to a report by the International Energy Agency, CCU has the capacity to capture and utilize an estimated 6.2 billion metric tons of CO2 emissions annually by 2050. This illustrates the ability of CCU to offer a more sustainable and circular approach to resource management, while simultaneously curtailing overall CO2 emissions. Such measures encompass the repurposing of industrial waste as sodium-rich sources, a reduced dependence on virgin materials, and the advancement of a closed-loop system for sustainable sodium carbonation pathways. The use of aqueous alkaline wastes with a high concentration of Na+, including desalination facilities reject brine 95, 96, saline brine from textile industry [97], or CO2storage sites [98], etc. provides a sustainable and efficient means of CO2sequestration due to its high capacity.
3.1. Modified Solvay process
Numerous desalination facilities face the challenge of identifying effective methods to manage or dispose of the substantial quantities of concentrated brine generated during the desalination operations. El- Naas et al. [64] examined the viability of the Solvay process as an innovative method for handling reject brine and CCU. In another study, Mohammad et al. [99] employed response surface methodology to maximize the CO2 capture by establishing the primary operating parameters using ammoniated saline. The CO2 capture efficiency attained at a maximum of 86%, while the Na+ reduction efficiency reached 33% under optimum conditions.
Although the traditional Solvay Process has already provided a suitable process to treat the problems of brine and CO2, this method still has some disadvantages. The biggest issue due to high volatility ammonia at any temperature which has an impact on environment and human health 100, 101. This limitation makes this procedure challenging to scale up. Researchers have investigated the use of stable alkanolamines as substitutes for ammonia in the traditional Solvay process, yielding promising findings. The interaction between amines and CO2 follows either the carbamate or bicarbonate pathway, depending on the amine type. Carbamates are produced by primary and secondary amines, while bicarbonates result from tertiary and sterically hindered amines [102]. When carbamate hydrolysis occurs, CO2 absorption capacity can exceed predictions. The presence of brine enhances CO2 uptake as NaHCO3formation increases. Equilibrium is reached when pH drops below 9 or when all amines converts to amine chloride, explaining the increased CO2 absorption efficiency in the presence of NaCl [103]. Huang et al. [104] reported notable results, with monoethanol amine (MEA) and methylaminoethanol (MAE) emerging as effective substitutes for ammonia, demonstrating a NaHCO3 yield of 0.0143 kg and an absorption capacity of 0.9 mole per mol MAE at specific concentrations. Dindi et al. 105, 106 evaluated the enhancement performance of several alkylamines, including 2‐ amino 2‐ methyl propanol (AMP), piperazine (PZ), MEA, and methyl diethanolamine (MDEA) for their capacity to improve the Solvay process by substituting ammonia. AMP has been recognized as the superior amine to achieve high precipitation yields under conditions of high brine concentration, medium amine concentration, and lower temperatures.
Although amines show promising results in the carbonation phase, a key limitation is the inability to regenerate amine-chloride formed during the process. Furthermore, the degradation of amines can lead to the release of harmful substances, potentially polluting the brine.
Other than amine solvents, different metal oxides such as CaO/ Ca(OH)2, KOH, and Al2O3 were examined to modify the traditional Solvay process. El-Naas et al. 107, 108 substituted NH3 in the conventional Solvay method by CaO, as described by Eq. (36).(36)
Under optimized conditions, a maximal 35% Na+ removal and 0.92 g CO2absorption capacity per gram of CaO were achieved. Using the industrial actual reject brine, which has a salt level of 63 g/L, and under optimum circumstances, it was possible to obtain a sodium removal rate of 43% [109]. Lee et al. [110]precipitated Ca (OH)2 from brine and applied it to the modified Solvay process. The CO2 capture was 0.6 mol/ L brine, and the Na production rate was 45% under Ca(OH)2 of 25 g/L and a temperature of 15 °C [110]. The conditions for the precipitation of NaHCO3 were consequently improved as the result pH stability at lower temperatures (10 or 17.5 °C) and increased basicity. This is because CO2dissolves in brine to form carbonic acid (H2CO3), which then decomposes into bicarbonate ions at a low pH (high temperature). However, Ca(OH)2 may react with CO2 to precipitate CaCO3 during the process, interrupting the combination of HCO3−and Na+ that can lead to clogging of equipment. Therefore, the reactor’s design or experimental parameters should be improved to increase the NaHCO3 production.
KOH is another alkaline agent, capable of greatly enhancing the effectiveness of CO2 absorption due to its high solubility in purified brine and its capacity to sustain at pH value of 13.6. The overall reaction of the KOH based modified Solvay method is illustrated in Eq.(37) [111].(37)
Mourad et al. [111] compared the CO2 absorption capacity of CaO/Ca(OH)2, KOH, and Al2O3. The KOH in the modified Solvay process could achieve a CO2uptake of 0.31 g CO2 CO2/g KOH, while ≈ 0.92 g of CO2 was captured by 1 g of CaO. On the other hand, using Al2O3 is discouraged due to its inability to capture CO2 effectively. This ineffectiveness is attributed to its limited solubility and relatively low initial pH level of ≈8.7 during the reaction [111]. In other studies, a response surface methodology (RSM) evaluation was implemented to predict and optimize the experimental factors, and there were two different predictions. The first predicted optimal conditions were gauge pressure of 2 bar, gas flow rate of 776 mL/min, and KOH of 30 g/ L. These conditions showed CO2 uptake of 0.5 g CO2 g/g KOH and a Na production rate of 45.6%. The second prediction was under a temperature of 10 °C, CO2 gas flow rate of 848.5 mL/min, gauge pressure of 2.1 bar, and KOH concentration of 110 g/L. The results revealed an optimum CO2 absorbance value of 0.58 g/g KOH and Na production rate of 44.1% 112, 113.
Dindi et al. [114] proposed metal oxide absorbent obtained by calcining Mg–Al layered double hydroxide (LDH) can be applied. LDHs are made of alternating layers of positively charged di or tri-valent cations and negatively charged balancing anions, which can remove chloride and generate hydroxide in the solution according to Eq.(41). The brine solution, which is a combination of NaCl and NaOH, is subsequently utilized to capture CO2 in order to produce NaHCO3. Furthermore, calcination allows the regeneration of the solvent. The regeneration process produces chemicals containing Cl¯, including Cl2 gas and HCl, as byproducts.(39)
The author concluded that the carbonated solution exhibited an approximate CO2 absorption capacity of 0.082 g CO2/g. The Na+ ions were reduced by 20%, and the process produced 0.044 kg of NaHCO3 /kg carbonated solution. Nevertheless, further examination is necessary regarding the application of mixed metal oxides, wherein the primary objective is to identify circumstances that optimize CO2 capture and NaHCO3 production in the modified Solvay process.
In addition to reject brine, other industrial alkaline wastes have been studied to promote pH and form NaHCO3 through reactions with CO2 which can stabilize and safely dispose of these environmental wastes using modified Solvay process. The utilization textile dye bath effluent to convert industrial flue gas to sodium bicarbonate is investigated by Krishnaveni and K. Palanivelu [97]. The maximum sodium ion (Na+) removal efficiency of 38% is achieved under the optimal reaction parameters. However, the absence of individual separation stages results in low purity of the product, which restricts its industrial application.
3.2. Mineral carbonation
Apart from the Solvay Process, mineral carbonation proved to be successful in generating carbonated goods of better purity and improving the efficiency of carbonation. Indirect CO2 carbonation technologies consist of two stages: an electrolysis stage, which involves the extraction of alkaline from brine, seawater, or industrial byproducts; and a carbonation stage, which involves the reaction of the alkaline with CO2 produced from emission sources in order to yield minerals 115, 116, 117. In specific instances, alkaline solution derived from naturally occurring minerals are employed directly [118], while intermediate substances utilized in the carbonation process are procured externally [27].
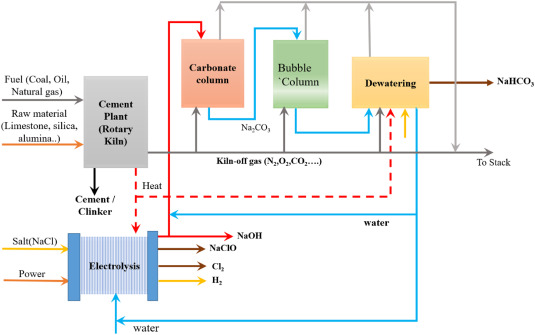
The Capitol Aggregate San Antonio Cement Plant in San Antonio, Texas, USA developed SkyMine® employs an indirect mineral carbonation process to produce NaHCO3. The plant utilized Sodium hydroxide NaOH solution that captures approximately 90% of CO2 from a flue gas split stream and produces hydrochloric acid, bleach, and sodium bicarbonate. The NaOH is obtained by the process of electrolysis of a sodium chloride (NaCl) solution [24]. Fig. 2 illustrates the schematic diagram of the SkyMine® process 119, 120. The initiative has generated 1.4 kilotons of sodium bicarbonate annually from 7.5 kilotons of carbon dioxide since its inception [121]. The SkyMine® technique exhibits a 30% reduction in energy consumption when compared to the conventional amine procedure for carbon capture.
 Fig. 2
Fig. 2Lee et al. [117] performed a bench-scale experiment to test the efficiency of the mineral carbonation process using NaOH and obtained a CO2 conversion rate of 95%. Furthermore, the resulting NaHCO3 has 97% or above purity, showing that it is suitable for industrial use. In addition, a specialized high-ion conductive membrane was utilized in the saline water electrolysis stage, which is the highly energy-intensive phase in the mineral carbonation plant. This implementation resulted in a minimum 8% reduction in electrolysis energy consumption [122]. A mineral carbonation plant at a pilot size was established using the findings from evaluating the performance of a smaller unit at a bench scale. However, the mineral carbonation technology proposed in this study would need significant cost reductions to be a viable alternative to CCS technology for large-scale CO2processing and greenhouse gas reduction.
In saline water containing high concentration of calcium and magnesium ions, the purpose of brine mineral carbonation by using pH-swing is to enhance the dissociation of CO2 by introducing alkalinity and separate the calcium and magnesium ions to get high-purity carbonated products and avoid the precipitation competition between CaCO3 and MgCO3. When calcium and magnesium components are separated, sodium cations and chloride anions are the most prevalent ions in the remaining solution that can be used to precipitate sodium bicarbonate. Kang et al. [12] employed NaOH as a pH adjuster for separating calcium and magnesium ions in seawater-derived effluent, while CO2was introduced in its ionic form using alkanolamine absorbents. The remaining NaCl solution was then subjected to react with CO2 to precipitate NaHCO3. According to the CO2 balance evaluation, NaHCO3 precipitation contributed the greatest CO2 uptake compared with CaCO3 precipitation and MgCO3precipitation. Kim et al. [123] developed a two-stage indirect carbonation of seawater- based industrial wastewater, which involves the separation of cations and conversion in to value added products. In the first step, the aqueous NaOH solution was fully saturated with CO2 and high-purity (92.2%) NaHCO3 was produced. In the next step, wastewater was pretreated with Na2SO4 to remove the Ca2+ ions to ensure the high purity of the metal carbonates in the second mineral carbonation step. The pretreatment resulted in Ca2+ free wastewater, which was used to produce MgCO3 ·3H2O in the second carbonation step. In addition, because NaOH can be obtained by seawater electrolysis, this process was considered as a practical method to mineralize CO2 as high-purity metal carbonates.
Moreover, other alkaline waste materials are utilized in mineral carbonation processes, which can help in reducing the overall process costs and minimizing negative environmental impacts. In a study by Na et al. [124], waste bittern from solar salt production was investigated for its potential use in magnesium (Mg) production and CO2 capture through a two-step process. Initially, they recovered the dissolved Mg2+ from waste bittern as Mg(OH)2 precipitates by adding NaOH. Subsequently, the dissolved sodium ions (Na+) in the residual waste bittern were recovered as sodium bicarbonate (NaHCO3) by reacting with CO2. They achieved >99% Mg precipitation from bittern at NaOH/Mg molar ratios of 2.70–2.75 and pH 9.5–10.0. Moreover, recovery of sodium bicarbonate precipitate resulted in the sequestration of 63 g CO2 per liter of bittern. In another study, Villardi et al. [125] assessed the capacity of produced water, which is a byproduct of petroleum extraction, to reduce CO2 and salinity requirements. The analysis revealed a sodium chloride conversion rate of 44.5% into sodium bicarbonate, which can potentially sequester 250,000 tons of CO2 annually. Additional research is necessary to analyze the impact of utilizing various forms of sodium-rich alkaline industrial waste to improve pH levels and generate valuable goods, hence enhancing the sustainability of this process.
3.3. Electrochemical CO2 conversion using saline wastes
Several studies investigated the utilization of saline wastes using electrochemical CO2 capture technology with different electrolytes. Rau et al. [126] investigated the conversion of silicate minerals into NaHCO3 as primary product, accompanied by silica, metal sulphate, and hydrogen in the presence of sodium sulfate electrolyte. The industrial deployment of electrolysis requires both flexibility and stability in order to effectively treat various saline wastes. Acosta-Santoyo et al. [127] combined electrolysis, reactive absorption, and crystallization to produce pure NaHCO3, hypochlorite, and hydrogen using alkali generated in the chloralkaline process to fix carbon dioxide in highly saline wastewater. The results show over 90% CO2 capture efficiency with an energy requirement of approximately 0.65 kWh per kilogram of CO2, making it promising for sustainability, particularly with the potential use of green energy sources.
Dara et al. [128] proposed a new configuration of the electrochemical cell that utilizes a platinum catalyst integrated that facilitated the conversion of CO2 into carbonate and bicarbonate salts. The electrochemical cell comprised five compartments, including the cathode, anode, acid, base, and saline feed solution, which were partitioned via cation and anion exchange membranes. It was found that possible catalyst poisoning was the cause of the substantial decline in cell efficiency that accompanied an increase in CO2. The electrochemical cell, consisting of five compartments, exhibited commendable flexibility, exceptional stability, and enhanced process control. Fig. 3(a) depicts a diagram of the electrochemical cell. In their subsequent study, Dara et al. 128, 129 conducted a comparison between the rate at which carbonic acid is formed and the rate at which CO2 is present without being converted into carbonic acid. It was shown that utilizing CO2 directly results in a product production flow that is 40 times greater than when using carbonic acid. The limited ability of CO2 to dissolve in water is addressed by employing a gas-fed electrode in the cathode compartment. This electrode facilitates the conversion of the gaseous mixture (CO2 and oxygen) and salty water into valuable products. Fig. 3(b) shows the technical visibility of the approach for the production of bicarbonate salts and acids.
 Fig. 3
Fig. 3The energy cost serves as the primary performance measure, quantifying the amount of energy that is wasted in the process of CO2 utilization. Thiruvenkatachari et al. [130] carried out economic evaluation of electrochemical CO2 utilization processes to reveal their impact on the post combustion process integrated with a 600 MW power plant. The method comprises the generation of electrical energy and the synthesis of NaHCO3 through a chemical reaction. This is achieved by establishing a pH difference between the electrodes using amine-CO2, sodium salt brine waste, and alkaline fly ash (Ca(OH)2) solutions. The integration process showed a reduction in CO2 foot print of approximately 6.4% and 17.8% for MEA and AMP/PZ systems respectively. In another study, an electrochemical CO2 mineralization approach also proposed for treating red mud waste from the aluminum industry [131]. The authors employed a system utilizes hydrogen-cycled membrane electrolysis to efficiently mineralize CO2, recover carbon and sodium resources, and produce high-purity NaHCO3. The system demonstrates high electrolysis efficiency, with an average efficiency of 95.3% and a purity of 99.4% for the obtained NaHCO3 product. The process operates at a low electrolysis voltage of 0.453 V at 10 mA/cm2, indicating potential for low energy consumption in industrial applications.
Although CO2 has a high solubility, there are limits to the process, such as the possibility of catalyst poisoning caused by CO2, leading to a decrease the performance of the electrochemical cell. Furthermore, membrane fouling and its expensive cost limit the practical applicability of this technology. The potential discharge of ions not only impacts the efficiency of the cell, but it may also lead to the formation of corrosive gases such as Cl2. These gases can corrode valuable electrodes, disrupting continuous operation and resulting in higher maintenance expenses [128]. It is necessary to examine the purity of NaHCO3and hydrochloric acid prior to expanding the production to an industrial level. Table 3 summarizes the process parameters for precipitating NaHCO3 using different sodium rich industrial wastes in CCU process.
| Method | Na+ Source | Reactants | Parameters | Reactor | Result | Ref |
|---|---|---|---|---|---|---|
| Modified Solvay carbonation | Reject brine | MEA/NaCl, MAE/NaCl, 10 % CO2 |
Temp = 25 °C pH = 9.5 |
Double stirred reactor |
Main product: NaHCO3 Max. CO2uptake capacity 0.92 mol CO2/mol of MAE |
[104] |
| Solvay carbonation | Reject brine |
NaCl, NH3, 99.9 % and 10 % CO2 |
Temp = 20 °C PH = >8 |
Bubble column |
Main product: NaHCO3 Na removal efficiency: 40–44% >90% Maximum CO2reduction |
[64] |
| Solvay carbonation | Textile dye bath effluent |
Textile effluent, NH3, 99.9 % CO2 |
Temp = 20°C CO2 gas flow rate= 1.8 L/min PH = 8.5 |
Plexiglas made |
Main product: NaHCO3 Na removal efficiency: 38 % |
[97] |
| Modified Solvay carbonation | Reject brine | Brine (NaCl), alkanolamines (MEA, AMP, MDEA, PZ), 15 % CO2 |
Temp = 40°C Pressure = 1 bar |
Stirred cell reactor |
Main product: 13 g NaHCO3/100 g-solution Na removal efficiency: 85% Max. CO2absorption capacity: >0.9 mol- CO2/mol-amine |
[106] |
| Solvay carbonation | Reject brine |
NaCl, NH3, 10 % CO2 |
Temp = 19 °C Pressure = 1 bar pH =11.2 |
Bubble column |
Main product: NaHCO3 Na removal efficiency: 33% CO2 Capture efficiency:86% |
[99] |
| Solvay carbonation | Reject brine |
NaCl, NH3, 99.9 % and 10 % CO2 |
Temp=25 °C | Bubble column |
Main product: NaHCO3 Na removal efficiency: >40% 99% reduction of CO2 in the exit gas. |
[132] |
| Solvay carbonation | Reject brine |
NaCl, NH3, CO2, |
Temp = ≥20 °C pH = 8 |
Packed column |
Main products: NaHCO3, CaCO3 8.1 wt% of the initial CO2effectively captured |
[133] |
| Solvay carbonation | Waste bittern from saltern | Bittern, NH4OH, 99.9 % CO2 |
Temp = 25 °C CO2 gas flow rate = 0.2 L/min |
Bubble column |
120 g of NaHCO3 precipitate per liter of bittern equivalent to 63 g CO2captured |
[124] |
| Modified Solvay carbonation | Reject brine |
Brine (NaCl), CaO 10 % CO2 |
Temp = 20°C–50°C Pressure =1 bar PH=>10 |
Bubble column |
Main product: NaHCO3 Na removal efficiency: 35% Max. CO2absorption capacity: 98% at 20 °C |
[107] |
| Modified Solvay carbonation | Reject brine | Brine (NaCl), Mg–Al–O sorbent, pure CO2 |
Temp= 25 °C Pressure=1bar pH=10 |
Bubble column |
NaHCO3 yield of 44 g/kg solution Na removal efficiency:20% 1.86 mol CO2/kg carbonated solution |
[114] |
| Solvay carbonation | Reject brine |
NaCl, NH3, 99.9 % CO2 |
Temp = 20°C˗ 38°C | Bubble column |
Main products: NaHCO3 , Na2CO3, NH4HCO3, (NH4)2CO3 CO2 Capture efficiency: > 91% at 20°C, 80% at 38°C |
[65] |
| Modified Solvay carbonation | Reject brine |
Brine (NaCl), KOH 10 % CO2 |
Temp=50 °C Pressure=2 barg PH= 13.6 |
Inert-particle spouted bed reactor |
Main product: Mixed solids (NaHCO3, CaCO3, KHCO3, K2CO3, and KCl) Na removal efficiency: 45.6% Max. CO2uptake capacity 0.50 g CO2/g KOH |
[112] |
| Solvay carbonation | Petroleum industry produced water | Produced water, NaOH, NH4OH |
Temp = 10°C Pressure= 2 bar pH=11 |
Random packing |
Main products: NaHCO3, Conversion of 44.5% of NaCl into NaHCO3and capture of 250,000 tons of CO2 per year. |
[125] |
| Modified Solvay carbonation | Reject brine |
Brine (NaCl), KOH 10 % CO2 |
Temp = 10°C –50 °C Pressure = 1-3barg PH= 13.6 |
Inert-particle spouted bed reactor |
Main product: Mixed solids (NaHCO3, CaCO3, KHCO3, K2CO3, and KCl) Na removal efficiency: 44.1% at 10°C Maximum CO2uptake value of 0.58 g/g KOH |
[113] |
| Modified Solvay carbonation | Reject brine |
Brine (NaCl), KOH, 10 % CO2, NH4HCO3 |
Temp = 10 °C Pressure = 2.1 barg |
Inert-particle spouted bed reactor |
Mixed solids (NaHCO3, CaCO3, KHCO3, K2CO3, and KCl) Na removal efficiency: 65% Max. CO2uptake capacity: 108.2 g-CO2/L |
[134] |
| NaOH carbonation | Brackish water | 10 wt% NaOH, 30 mol% CO2 | Temp=25–40 °C | Absorber and a bubble column | Main Products: Purity NaHCO3product (> 99 wt %), H2, Cl2, and fresh water | [135] |
| NaOH carbonation | Desulfurization waste | Na2SO4, NH3,CO2 |
Temp = ambient NH4OH= 24 ml Pressure = 7 bar |
Agitated reactor |
Main product: NaHCO3 = (85.9–92.3%) purity Yield = 46.64–58.2) |
[136] |
| NaOH carbonation | Black liquor waste of paper industry | Hyroxide/ from black liquor/, 30 Vol % CO2 |
Ambient pressure and temperature Gas flow = 200 mL/min pH=11.64–8 8 |
Stirred glass reactor |
Absorption capacity is 30 g of carbon dioxide/L of black liquor At the final pH of 8, bicarbonate ions were the most abundant species |
[137] |
| Electrochemical | Saline water | 0.25 Na2SO4/electrolyte |
Temp= Ambient Pressure= 1 atm pH= 11.1 |
Electrolysis cell |
Main products: NaHCO3, silica, metal sulphate, and hydrogen. Energy-efficient (<300 kJ/mol of CO2captured) |
[126] |
| Electrochemical | CO2, Na2SO4,and carbide slag | Na2SO4/electrolyte |
Gas flow rates= 20 mL/min Temp= 25°C, 45°C |
CO2mineralization cell |
A metric ton of CO2 could produce 1.9 t of NaHCO3/99.4% purity/, generate 137.3 kW h of electricity, and consume 1.23 t of carbide slag. |
[138] |
| Electrochemical | Reject brine | 0.1 M H2SO4/electrolyte |
Liquid flow rate= 5 ml/min Gas flow rates= 50 ml/min Pressure=1 atm Temp= 20°C |
Five compartments electrochemical cell |
Main Products: NaHCO3 and HCl The average product fluxes for HCl and NaHCO3 were 0.05 mM/cm2h |
128, 129 |
| Electrochemical | Brine and alkaline fly ash | AMP/PZ |
Pressure=1 atm Temp= ambient |
CO2mineralization reaction cell |
Main Products: NaHCO3, Electricity generation Reduced the CO2 footprint by about 17.8% (429.92 kg CO2/t CO2processed) for the AMP/PZ system in 600 MW coal fired power plant |
[130] |
| Electrochemical | Leached red mud alkaline waste | 0.5 M H2SO4 |
Temp=30 °C. Flow rate CO2= 100 ml/min |
Hydrogen-cycled membrane electrolysis |
Main product: NaHCO3(purity 99.4%), Average electrolysis efficiency is 95.3%. |
[131] |
4. Waste minimization and recovery
Waste minimization and valorization create a circular flow of resources within the production system. The traditional Solvay method uses calcium oxide to regenerate NH3, and recycle it for absorption which necessitates further advancements [139]. This is detrimental for the CO2 utilization technology since the synthesis of calcium oxide synthesis is CO2-intensive. Limestone calcination releases one mole of CO2 for every two moles of CO2 absorbed by power plants, which limits the application of the Solvay process in CO2 utilization industry 100, 140. On the other hand, a large amount of ammonium chloride (NH4Cl) a byproduct of ammonia soda process is released into the environment as a waste 141, 142. Due to its role as a contributor to ammonia nitrogen pollution, NH4Cl has resulted in several environmental issues [143]. Evaporation [144] and crystallization [145] are processes that applied to eliminate NH4Cl. However, the generation of secondary salt and high expenses are the disadvantages of these processes 142, 146.
Huang et al. [104] introduced a technique to regenerate ammonia from ammonium chloride by utilizing activated carbon instead of CaO, thereby eliminating the need for CaO in the ammonia recovery process. In another study, de Carvalho Pinto et al. 133, 147 proposed Ca(OH)2 (lime milk) from steel slag reacted with aqueous NH4Cl to recover a significant portion of NH3, which can be recyclable in the process. They found that in the NH3 recycling process, the lime milk is partially replaced by steel slag that could recover almost 40 wt% of the total ammonia. Recently, electrochemical technology has been proposed as a promising alternative way to recover NH3 from saline wastewater 148, 149. Öner et al. [150] used bipolar membrane electrodialysis to recover NH3⋅H2O from mixed salt wastewater of NH4Cl and NaCl. In this case, CaO is unnecessary, leading to the absence of liquid waste, which is primarily composed of CaCl2 and NaCl. They found that the desalination ratio exceeds 90%, the salt based acid and base conversion ratios fall within the range of 60% and 80%, and the energy consumption of the bipolar membrane electrodialysis process ranges from 1.54–2.33 kWh/kg acid. The bipolar membrane electrodialysis process provides the benefits of removing salts from water/ desalination, and the formation of NH3⋅H2O that can be recycled to capture CO2 as shown in Fig. 4. However, the drawbacks of bipolar membrane electrodialysis arise from NH3 loss and energy expenditure, which are caused by co-ion transition, reverse diffusion, and electro-osmosis. Therefore, further research is necessary to enhance the structure of ion exchange membranes in order to alleviate the constraints on mass transfer. Therefore, both energy efficiency and product yield can be improved.
 Fig. 4
Fig. 4The utilization of byproducts from ammonia sodium sulfate carbonation as a waste reduction approach represents a compelling strategy that aligns with the principles of the circular economy, rendering the technology potentially feasible. Bichel and Schaaf [70] proposed the use of sodium sulfate as an alternative raw material that facilitate the generation of secondary output (ammonium sulfate and/or calcium sulfate). Ammonium sulfate can be utilized in the fertilizer industry either in its aqueous state, as produced from the reactor, or it can be crystallized to produce more complex fertilizers. However, there is a lack of scientific investigations that assess the crystallization and purification of NaHCO3 concurrently with the production of (NH4)2SO4 using Na2SO4, NH3, and CO2 as raw materials in a technical and cost-effective manner [34]. The evaluation of this manufacturing pathway with respect to soda ash production was conducted using the commercial process simulation software Aspen Plus®. The results confirmed certain anticipated or known technical characteristics: the utilization of NH3 to increase productivity may necessitate greater quantities of water during the purification stages, which could prove problematic given the increased energy and electricity consumption associated with the recycling process [151].
5. Modelling and simulation studies
It is generally accepted that the development of precise, comprehensive, and robust plant design modelling is necessary to analyze different operational parameters in a manner that does not put the actual system plant at risk, saving a lot of time and cost. The preferred model should be rigorous and capable of handling all parts of the process while also being simple enough to be readily applied [152]. Maharloo et al. [55] proposed a new configuration of an industrial bubble column for producing high-purity sodium bicarbonate crystals. They developed a computational model involving three-phase mass balance equations to investigate and compare with a conventional bubble column reactor. The configurations of the bubble columns are shown in Fig. 5 (a)(b). The model incorporates the use of the Gauss-Newton method in MATLAB programming based on the backward finite difference method and Danckwerts theory to predict CO2 diffusion and absorption. As shown in Fig. 5 (c), the application of the new reactor configuration improves solid production significantly, leading to a 50% increase in which the model is in good agreement with industrial data, with annual production capacities of 20000 and 30041 ton/year for conventional (case I) and new configuration bubble column (case II), respectively. Moreover, plant data analysis and modeling results reveal that by recycling CO2 in the new configuration, 314 mol/m−2 s−1 CO2 is saved in each loop. the CO2 mole fraction in the gas phase thought the column in new configuration is more than that of conventional process (see Fig. 5 (d)).
 Fig. 5
Fig. 5Yoshi et al. [28] compared sodium carbonation routes such as soda ash, Solvay and sodium sulphate carbonation process for the optimization of NaHCO3production through CO2 utilization. They evaluated the process using Aspen plus simulation software and optimization techniques. The result showed that soda ash carbonation emerging as the most favorable option at 35°C, generating a gross profit of US$ 0.23/kg. In another study, Wang et al. [77] conducted a technoeconomic analysis using Aspen Plus to investigate the co-production of high-purity CO2 and NaHCO3 in cement industry using ammonia-sodium sulphate process. The procedure entailed the utilization of methyldiethanolamine (MDEA) for the absorption and desorption of CO2, NH3, and Na2SO4, followed by the carbonization process. Utilizing a saturated Na2SO4 solution boosted the absorption of CO2, resulting in an increased CO2 usage rate when the Na2SO4concentration was raised. The maximum yield of NaHCO3 was achieved at a crystallizer temperature of 35.5°C, whereas the yield decreased as the crystallizer temperature increased. When the pressure for crystallization was raised from 1.0 bar to 2.55 bar, the flow rate of NaHCO3 exhibited a slow increase from 5.60 kmol/h to 5.63 kmol/h in response to the higher reaction pressure. Hence, the influence of crystallizing pressure on the reaction was negligible. In addition, the author ascertained that the economic evaluation of the undertaking yielded a net return of 13.51% after 6.48 years, providing a means to mitigate CO2 emissions while also yielding economic advantages.
Mio et al. [153] formulated a detailed rate-based model for CO2 removal using the NaOH process focusing on CO2 absorption systems employed in the steel-making industry. They used Electrolyte NRTL thermodynamic model and compared the absorption of carbon dioxide by MDEA, membranes, or sodium hydroxide to produce sodium bicarbonate from a life cycle viewpoint. The utilization NaOH to convert CO2 in to NaHCO3 found to be the most favorable option in CCU. Caudle et al. [83] introduced an innovative method for optimizing process models that combines manufacturing of glass and CO2 mineralization in order to generate NaHCO3 from CO2 using NaOH, thereby significantly reducing net emissions. The author simulated in AVEVA PRO, and OLI aqueous electrolyte thermodynamic model was used to analyze CO2 emission in three cases, and in each case, CO2 is captured and proceeds direct conversion into NaHCO3 by using NaOH solution. The mineralization scenarios exhibit net negative carbon dioxide emissions ranging from 152 to 528 kg CO2 per of ton glass, in comparison to the unabated glass process and the conventional Solvay process utilized for Na2CO3.
6. Enhancement technologies of sodium carbonation pathways
6.1. Solvent development
The primary objective in the development of novel solvents is to enhance energy efficiency, increase stability against oxidative and thermal decomposition, and reduce emissions [154]. It has been demonstrated that the modified Solvay route could potentially reduce total equivalent work by 70% and operation energy by 30% when compared to the traditional Solvay process [106]. For example; the thermodynamic study showed that development of Ca-based Solvay process emerged as more spontaneous and effective, demonstrating superior performance in CO2 capture and energy consumption than the standard NH3-based Solvay process. This finding underscores the significance of exploring innovative processing routes and materials in solvent development. One intriguing avenue for future research lies in the exploration of solvents capable of reducing the solubility of NaHCO3 while maintaining high reaction rates. By investigating alternative chemical routes, optimizing CO2 utilization, and leveraging thermodynamic analyses, researchers can further refine solvent development strategies to align with sustainability goals and industry demands.
6.2. Process intensification
Process intensification refers to the incorporation of several unit activities inside a process or the new development of process units, with the aim of reducing equipment size and cost, minimizing emissions, and enhancing productivity. Process intensification techniques can be categorized as intensified equipment and intensified methods 155, 156. The design and configuration of a reactor play a crucial role in enhancing the reaction kinetics and internal hydrodynamics of processes, while also reducing costs and increasing energy efficiency in the production of NaHCO3. Researchers developed and investigated a novel Inert Particle Spouted Bed Reactor (IPSBR) to address CO2 capture and reject brine management in the Solvay process [107]. The study reported an impressive CO2capture efficiency of up to 97.7% with a gas-to-liquid volume ratio of 123 [107]. The increased effectiveness of CO2 capture and ion removal was a result of the rotary movement exhibited by the particles within the reactor [157]. Validation of the results was performed using a bench-scale IPSBR, showing satisfactory results between experimental findings and modelling analysis 35, 158, 159.
According to different studies, bi-carbonation occurs at a smaller rate compared to carbonation during production of NaHCO3[117]. To solve this, Shim et al. [18]developed distinctive continuous reactor design, as shown in Fig. 6, of an integrated a bubble column connected to structured packing bed reactor. The researchers investigated the effectiveness of CO2 capture at room temperature reacted with NaOH solution generated through NaCl electrolysis. A maximum of 95% CO2 capture and 97% NaHCO3 generation were achieved. However, the process has drawbacks due to its high energy expenditure and complex reactor design [35]. Similarly, Lee et al. [117] employed a series connection of two columns to compensate for the comparatively sluggish pace of the bi-carbonation process. After carbonation occurred in the packed column, which is the initial reactor, bi-carbonation took place in the bubble column. However, the kinetics study which greatly contributes to increasing and boosting the reaction rate, has not been well examined. This can also facilitate the expansion of this method to the industrial scale.
 Fig. 6
Fig. 6Zhang et al. [160] has made additional progress by utilizing catalytically active nickel nanoparticles (NiNPs) within a tubular reactor with continuous plug-flow. The reactor, featuring a helically coiled tube, facilitated controlled and efficient mixing without the need for mechanical agitation. It allowed easy optimization, integration, and scalability for industrial applications, accommodating both homogeneous and heterogeneous reactions for continuous production.
Membrane technology is a viable alternative to packed columns which has recently played a crucial role in the advancement of modern CO2 separation technology. Membrane contactors provide several benefits, including high interfacial area, operational flexibility, simple scaling, and independent gas and liquid operation [161]. Thus, they are able to offer a larger mass transfer and accomplish a quicker rate of mass transfer in the carbonation process [162]. Hwang and his colleagues introduced a novel approach employing hollow fiber modules with highly permeable membrane to directly utilize CO2 from the flue gas, aiming to reduce initial expenditure by a minimum of 30% [163]. Nevertheless, the accumulation of substances on the membrane surface and the saturation of pores from post-combustion flue gas components inhibit the efficiency of the membrane devices. Further research is necessary to address this issue [164].
6.3. Application of Catalyst
A highly effective additive/catalyst could help to accelerate the reaction rate, lower temperature threshold, and promote yield. When choosing promoters for the precipitation process, it is widely acknowledged that chemical properties such as rapid absorption rate, the chemical effect on the aqueous solution on decreasing the solubility of NaHCO3 and could increase its supersaturation tendency should be prioritized. Carbonic anhydrase (CA) is among the most promising catalysts capable of accelerating the CO2 bi-carbonation reaction by a significant amount [165]. Fig. 7(a, b) illustrates the structure and CO2 catalyzing mechanism of CA. It catalyzes the reversible hydration of CO2 to
 Fig. 7
Fig. 7Nevertheless, despite the remarkable potential of CA to absorb CO2 and operate as a catalyst, its practical implementation in commercial facilities is impeded by reduced catalyst activating and strength, which prevents its sustained utilization. In addition, there are concerns about high production costs and the possible depletion of the CA during carbonation process. Consequently, other investigations are endeavoring to address these problems [171].
Organic promoters, such as alcohols and polyols, can enhance absorption rates by virtue of the hydroxyl groups in their molecules, which have the ability to establish hydrogen bonds. Czaplicka et al. [172] studied the improvement of CO2capture using organic compounds such as neopentyl glycol, glycerol, isopropanol, methanol, pentaerythritol, and ethylene during the synthesis of CaCO3 by carbonation method. The results revealed that found that neopentyl glycol showed the highest carbon capture efficiency (86.1%). On another study, Chiang et al. [173] indicates that adding glycerol to an aqueous solution of NaOH can increase the overall chemical absorption efficiency of CO2 by over 90%. By increasing the rotation speed from 600 rpm to 1500 rpm, it was observed that the mass transfer coefficient increased by a factor of five and the absorption percentage and enhancement factor were doubled or tripled, respectively. Mun et al. [174] investigated the mineral carbonation process that results in the formation of sodium bicarbonate (NaHCO3) by carbonating 3 g of an aqueous solution containing dissolved ethanol and sodium hydroxide (NaOH), with the ethanol concentration varying between 50.5% and 97%. The production of NaHCO3 precipitates in this area increases parabolically with the ethanol concentration.
Another type of suitable organic promoter studied to enhance the rate of absorption in the modified Solvay process are amino acid salts 175, 176, 177. They are inexpensive, safe for the environment, have high absorption rates, and lower vapor pressure [178]. Amino acids are organic compounds containing amine(−NH2) and carboxyl (−COOH) functional groups, along with a side chain (R group) specific to each amino acid. There are 20 different types. Based on their stereochemistry, amino acids are defined by D and L enantiomers. The 20 amino acids are L-isomers, and their enantiomeric D-isomers are rarely found in nature [179]. Govindaraj et al. [176] employed amino acids (Glycine, L-arginine, and L-alanine) to improve the standard Solvay method and thereby boost the efficiency of Na+ recovery from textile dye bath effluent to produce NaHCO3. Arginine showed highest performance in conversion efficiency while also lowering the process’s ammonia need. However , although adding amino acid salts improved KG, the raising of NH3 vaporization is the drawback of this application 175, 180. Further research is needed explore new catalysts and mixed solvents that can enhance the bicarbonation reaction and required to improve the process for high CO2 capture from exhaust gases and production of pure NaHCO3.
The addition of inorganic compound can also accelerate the CO2 absorption rate. Recently, a very small amount (30 ppm) of nickel nanoparticles was utilized which resulted in carbon capture efficiency of 96.7%, in comparison with the case without catalyst [160]. The use of nickel nanoparticles led to a carbon dioxide gas utilization efficiency of 96.7% compared with only 87.8% without a catalyst. Although an inorganic heterogeneous catalyst can enhance the absorption rate, an analysis of solid particles with the presence of inorganic salts reveals the existence of small quantities of these metal ions in the final product, which is unfavorable for its future uses [181].
6.4. Renewable energy integration
The mismatch between energy supply and demand has resulted in significant emissions from power plants with increased energy production. To address this, a focus on capturing and storing greenhouse gas emissions is crucial, especially through renewable energy sources, for effective and economically viable emission reduction. The United Nations' thirteenth sustainable development goal aims for zero emission fuels by 2050, a target achievable through the utilization of renewable energy resources as highlighted in reference [182].
Bonaventura et al. [54] conducted an economic analysis of an integrated soda ash carbonation process facilitated by renewable energy sources for the synthesis of NaHCO3. In terms of energy, utilizing solar energy for heating in the plant resulted in 3% decrease in economic efficiency. The study concluded that sodium bicarbonate production through this process is feasible, utilizing inexpensive and abundant trona as a raw material, solar energy for heating, and enabling carbon dioxide capture and reuse with minimal emissions. Furthermore, investigations reveal that by retrofitting the soda ash carbonation process to a 600 MW coal-fired power plant, a local minimum energy penalty of 3.99% at a 90% CO2 capture efficiency and 99.9% CO2 outlet purity could be achieved [57].
As mineral carbonation uses electrolysis for the production of alkali agents, the cost of electricity is a hindrance to near-term commercialization. Using a renewable source, such as solar energy, the cost of electricity for electrolysis could be reduced, thus the CCU process can be operated cheaply. For example, 1 kg of MEA costs US$2–4, and this “cost problem” can be overcome by electrolysis using renewable energy. On the other hands, 1 kg of NaOH cost US$ 0.2–0.3 [183]. However, substantial cost reductions and advance technology development are necessary for the mineral carbonation technology to become techno-economically feasible alternative to CCS technology for large-scale CO2processing and greenhouse gas reduction.
7. Conclusions and future prospects
Several advances have been made to incorporate chemical precipitation to enhance the effectiveness of carbon capture and sequestration. Among so many options, sodium bicarbonate emerges as a potentially advantageous and renewable product to precipitate CO2 as thermodynamically stable carbonates, providing an effective and sustainable way to sequester CO2 that fits within the circular economy. Moreover, the exploitation of CO2 with sodium-rich wastes is notably appealing among several solutions suggested to mitigate the issue of industrial wastes, since it effectively addresses two environmental concerns simultaneously. The main approaches of sodium carbonation routes to precipitate NaHCO3 in carbon dioxide utilization process are summarized in the present paper, and prospects of these technologies were reviewed.
The soda ash carbonating process is one of the preferable ways for the production of NaHCO3 alone, due to its straightforward purification stages. There is a growing interest in advancing this technology towards the progression through additional research on aspects such as kinetic model studies, sorbent deactivation, equipment design, and byproduct utilization. However, because this method is employed by major chemical enterprises situated in close proximity to extraction regions and nations where transportation expenses do not pose a risk to the process, market circumstances and requests for additional coproducts play a crucial role in guiding decision makers to select more favorable alternative courses.
Ammonia is identified as the most suitable and cost-effective absorbent for sodium bicarbonate precipitation in brine solutions. However, limitations such as secondary waste generation and ammonia escape have hindered its widespread use in CCU applications. The integration of Solvay process for production of industrial-grade NaHCO3 with the CO2 utilization process, combined with NH3regeneration through a bipolar membrane electrolysis system, could have the potential to overcome the limitations of traditional carbon utilization methods, such as low efficiency and high costs. Future studies should focus on assessing the techno-economic feasibility of this integrated approach.
Mineral carbonation, comprising carbonation and electrolysis, is a promising technique for carbon capture and utilization and obtained high purity products However, the technology faces a significant bottleneck in the form of high electrolysis costs, hindering the commercialization of the process. To address this research and development efforts should concentrate on exploring and developing advanced electrolysis technologies that are more efficient and cost-effective. New reactor designs that can enhance the carbonation rate is also required future studies.
The sodium sulfate carbonation route offers an alternative method for producing secondary products such as ammonium sulfate which can be used as for fertilization industry. However, there is a scarcity of scientific literature on the evaluation of NaHCO3 crystallization and purification in conjunction with (NH4)2SO4 synthesis, in a cost-effective and technically thorough manner. Simulation and optimization approaches are useful tools that may be used to assess the feasibility of procedures from both technical and economic perspectives on a commercial scale.
The electrochemical conversion of sodium-rich sources has the potential to decrease the total capital and operational expenses associated with carbon capture and utilization. The practical feasibility of using this technique is limited by its high cost and susceptibility to membrane fouling. Further research is necessary to conduct experiments on several operational factors, including the electrochemical cell design, electrode design, reactant concentrations, cycle time, and reaction kinetics to optimize the synthesis of bicarbonate.
The potential of the discussed CO2 utilization routes is evident; however, further research is imperative to achieve an optimal process design. In the context of the carbonation process, ongoing research endeavors aim to develop highly efficient catalysts and reactors to address the challenge of the relatively low carbonation reaction rate. Moreover, high-concentration saline water for carbon capture in product preparation significantly impacts technical and economic efficiency. Future research should prioritize the separation and purification of solid product impurities, as well as the purification of flue gas impurities to enable the industrial-scale production of sodium bicarbonate. Due to the aforementioned issues, the number of techno-economic analyses conducted on the proposed methodologies is restricted. Furthermore, the environmental appeal of these processes can be regarded as the paramount criterion for capitalizing on them in the marketplace, as they enable the generation of carbon credits while also contributing to carbon mitigation scenarios.
Qiuxia Zhu: Visualization, Software. Gedion Tsegay Hailu: Writing – review & editing, Visualization. Jianfu Zhao: Supervision, Funding acquisition, Conceptualization. Yangyuan Zhou: Writing – review & editing, Supervision. Marta Sibhat Baraki: Writing – original draft, Investigation, Formal analysis, Conceptualization. Ningzheng Zhu: Writing – review & editing. Guodong Yao: Writing – review & editing, Supervision. Kaiyu Fang: Writing – review & editing
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Data availability
No data was used for the research described in the article.
© 2024 The Authors. Published by Elsevier B.V.