1. Introduction
The field of bioengineering focuses on the application of biological principles to generate economically useful products. Bioengineering is needed for medical devices, diagnostic tools, biocompatible materials, recyclable bioenergy, agricultural engineering, and more. The aim of bioengineering is to rebuild or modify biologic systems in order to generate marketable products in the fields of biotechnology, microbiology, biocatalysis, and others. Tissue engineering is not only related to human (or animal) tissue replacement, but also to plant tissues. Furthermore, the pharmaceutical sciences include engineering technologies to produce chemical drugs and recombinant proteins (i.e., therapeutic antibodies). This novel field has been termed pharmaceutical engineering.
Since time immemorial, humans have used plant products as sources for pharmaceuticals, agrochemicals, and nutrition. Even today, almost all of the world’s population depends on plant-derived products. In recent times, biotechnology offers attractive opportunities for the production of plant-based in vitro systems (e.g., callus cultures, cell suspension cultures, and organ cultures) and for genetic manipulation to facilitate the generation of desired plants and plant products. As an increasing number of natural habitats are rapidly being destroyed, biotechnological in vitro techniques may help to counteract the extinction of endangered species.
Plants adapt to abiotic and biotic stresses using their astonishing plasticity to remodel themselves [1] and by the generation of secondary metabolites that are activated by elicitors and released as defense responses [2]. The generation of chemical compounds from secondary metabolism can be induced by external stress signals (e.g., pathogen elicitors, oxidative stress, wounding, etc.), which are internally mediated by jasmonate, salicylic acid, and their derivatives as signal transducers [3]. These elicitor molecules stimulate defense or stress-induced responses in plants. These can be derived from the pathogens themselves (exogenous elicitors; e.g., chitin, chitosan, and glucans) or are released by plants by the action of the pathogen (endogenous elicitors; e.g., pectin, pectic acid, cellulose, and other polysaccharides) [4]. In contrast to these biotic elicitors, there are also abiotic elicitors that act as physical agents (i.e., cold, heat, UV light, and osmotic pressure) and chemical agents (i.e., ethylene, fungicides, antibiotics, salts, and heavy metals). Elicitors modulate gene expression in response to chemical and physiologic stimuli [5]. They also induce enzyme synthesis, and thereby promote the formation of numerous secondary metabolites such as flavonoids, alkaloids, terpenoids, thionins, phenylpropanoid, and polypeptides [6].
By chance, many secondary metabolites not only reveal protective functions, but also possess medicinal value for human beings. Therefore, plant cell cultures represent interesting sources for the easy and scalable production of secondary metabolites. Approaches have been developed to optimize culture conditions and increase the yield of secondary metabolites in in vitro plant cultures. Furthermore, genetic manipulation of economic plants such as wheat, rice, maize, and others have led to stress- and disease-resistant varieties [7]. Hence, plant biotechnology may supplement traditional agriculture on an industrial scale [8].
Plant tissue culture represents an important technique in basic science and commercial application. In all major families of terrestrial plants, wounded tissue is recovered by non-differentiated callus cells. These callus cells can be cultured in vitro for biotechnological applications. Almost any part of the plant can be used to generate callus cultures. Explants taken from plant tissues slowly grow in vitro into a cell mass that ranges from amorphous and colorless to pale-brown, if they are obtained under sterile conditions avoiding microbial infection and cultured on solid gel medium supplemented with growth hormones (i.e., auxin, cytokinin). By passaging the cells regularly, callus cultures can be indefinitely maintained in vitro. Differentiated plant cells and cultured callus cells differ considerably. Callus cells are similar to non-differentiated meristemic cells; they reveal only small vacuoles and lack chloroplasts for photosynthesis, among other features. Callus cultures can re-differentiate into entire plants, if maintained under appropriate growth media that differ from standard culture media. While some callus cultures need dark growth conditions, others grow under specific day-night conditions (e.g., 16 h light, 8 h dark). Callus cultures usually grow at (25 ± 2) °C. They can be distinguished between cultures that grow in a rather compact form, and those that are friable. Friable callus cultures can be used to generate single-cell cultures that are maintained in slowly shaken liquid medium.
Plant tissue cultures can be traced back to Gottlieb Haberlandt (1854–1945), who established the first callus root or embryo cultures at the beginning of the 20th century [9]. During the 1940s and 1960s, technical advancements led to the further development of plant tissue culture techniques in order to investigate cell behavior (including cytology, nutrition, metabolism, morphogenesis, embryogenesis, and pathology), the generation of pathogen-free plants, and the conditions of germplasm storage and clonal propagation. Since the 1960s, the biosynthesis of secondary metabolites has become a subject of interest. With the advent of gene-based technological methods, novel applications were developed for callus cultures and other plant tissue technologies [10]. Plant cell cultures represent an effective means for the bioreactor-based large-scale production of therapeutically relevant secondary metabolites (e.g., anticancer drugs) [11].
The major advantages of cell culture systems, as compared with conventional whole-plant cultivation, include the following: ① The plant compounds of choice can be generated independently of external factors (e.g., soil composition or climate); ② cultured cells are not threatened by the attacks of microorganisms or insects; ③ cells of any plant—even rare or endangered ones—can easily be maintained in order to produce their secondary metabolites; and ④ robotic-driven regulation of secondary metabolite production decreases costs and improves productivity.
2. Callus formation
It is well known that stem cells from animal tissues usually differentiate into finally terminated tissue cells. However, it is assumed that differentiated tissue cells in plant tissues are capable of de-differentiating and regenerating wounded tissue or even the entire plant; it is also assumed that they can form totipotent callus cells [12], [13], [14], [15]. A more recent concept claims that plant cells do not re-differentiate, but that callus is rather formed from pre-existing stem cells [16], [17].
The underlying molecular modes of action that lead to stem cell differentiation and/or the differentiation–dedifferentiation of somatic plant cells are not completely understood. Stem cell-related genes are crucial for dedifferentiation processes. Their expression is not only regulated by transcription factors, but also by epigenetic events such as histone modification and DNA methylation[18].
Arabidopsis thaliana (L.) Heynh serves as a model organism for a wide range of diverse botanical investigations. In these plants, gradients of the phytohormone auxin in embryonic callus lead to the induction of stem cell formation by regulation of the PINFORMED1 (PIN1) protein [19].
The kinetics of the spatial and temporal distribution of hormonal and developmental meristem regulators has been investigated by microscopic live-cell imaging. Relevant determinants for growth include microtubules, transcription factor networks, and cytokinin pathways, which control WUSCHEL (WUS) expression, the auxin-mediated positioning of new primordia, and so forth [20].
Numerous transcription factors control meristem formation and dedifferentiation. The transcriptional repressors WUS and WOUND INDUCED DEDIFFERENTIATION (WIND) are driving forces to maintain stem cell totipotency, while TEOSINTE BRANCHED1/CYCLOIDEA/PROLIFERATING CELL FACTOR (TCP) is a transcriptional activator that inhibits stem cell totipotency in the shoot meristem [21].
In a time–kinetic study of immature embryo explants from maize (genotype A188), transcriptome-wide RNA-sequencing has been performed [22]. The expression of stress-related genes (glutathione-S-transferases and germin-like proteins) and hormonal transport genes (pin) was increased by more than eight-fold. Furthermore, genes related to the embryogenic growth initiation (e.g., transcription factors and receptor-like kinases) were also upregulated in a time-dependent manner. The synopsis of genes that were differentially regulated during the time course under study made it possible to build a model of the coordinated gene expression pattern in order to better understand the early steps of embryogenic culture initiation in maize [22].
3. Culture conditions
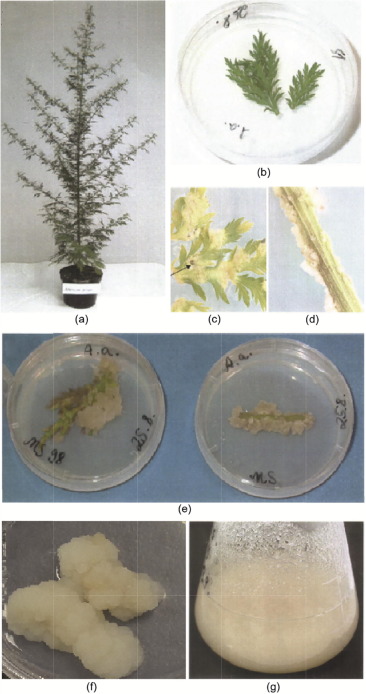
Although the stem cell concept is strongly considered for callus cultures, callus cultures do not develop from isolated individual cells, but from heterogeneous structural tissues (Fig. 1). Nevertheless, callus cultures are homogeneous enough to allow micropropagation for the generation of identical copies of plants with desired features. The laboratory conditions to maintain callus cultures differ from species to species, and need to be elaborated in each individual case. An example of callus culture generation is given in Fig. 1. External factors such as light, temperature, the pH of the medium, and the aeration of cultures affect secondary metabolite biosynthesis. Usually, callus cultures are maintained on solid agar medium supplemented with specific nutrients, salts, vitamins, and elements (e.g., nitrogen, phosphorus, and potassium). In general, high ammonium ion concentrations inhibit secondary metabolite formation, while the lowering of ammonium nitrogen increases it. Inorganic phosphate is essential for photosynthesis and glycolysis. High phosphate levels often promote cell growth and primary metabolism, while low phosphate concentrations favor secondary metabolite formation. Many secondary metabolites are formed by phosphorylated intermediates, which subsequently release the phosphate; examples include phenylpropanoids and terpenoids.
 Fig. 1. Generation of callus cultures from Artemisia (A.) annua L. (a) A. annuaplant; (b) A. annua leaf placed in MS98 medium; (c, d) callus growth in leaves and stipes of A. annua after 12 d, where the arrow in (c) indicates necrotic tissue; (e) callus growth in leaves and stipes after 19 d; (f) permanent callus culture; (g) callus single-cell suspension of A. annua in MS fluid medium. MS: Murashige and Skoog.
Fig. 1. Generation of callus cultures from Artemisia (A.) annua L. (a) A. annuaplant; (b) A. annua leaf placed in MS98 medium; (c, d) callus growth in leaves and stipes of A. annua after 12 d, where the arrow in (c) indicates necrotic tissue; (e) callus growth in leaves and stipes after 19 d; (f) permanent callus culture; (g) callus single-cell suspension of A. annua in MS fluid medium. MS: Murashige and Skoog.In general, the addition of precursors to the medium enhances product formation. The biosynthesis of secondary metabolites in plant cultures is usually low and needs to be enhanced in order to meet commercial purposes. The addition of precursor molecules to the medium frequently increases the product formation. The biosynthesis of most secondary metabolites consists of multistep reactions of several enzymes. Any step of the reactions in enzymatic biosynthesis chains can be stimulated to enhance product formation.
Typical culture media are well established, such as the Murashige and Skoog (MS) medium, White's medium, and the woody plant medium [23], [24], [25]. In most cases, specific phytohormones have to be added to the medium to stimulate callus growth. To optimize secondary metabolite production, a two-medium approach is desirable: one medium for good cell growth and another for good secondary metabolite formation.
Callus formation, or somatic embryogenesis, is driven by plant hormones such as auxins, cytokinins, and gibberellins. The regeneration of whole plants from callus tissue is called organogenesis or morphogenesis. For this process, specific hormones are required as well. The similarities of hormone-starch ratios in the callus compared with the corresponding ratios in plants represent an important determinant for embryogenesis and organogenesis [26]. Growth factors such as methylglyoxal and ascorbic acid enhance insufficient organogenesis in vitro[27].
Both embryonic and post-embryonic developmental programs regulate reprograming, totipotency, and differentiation by the genetic and epigenetic mechanisms of explanted tissues and callus formation under the influence of phytohormones [28]. Callus cultures can be either embryogenic or non-embryogenic. Embryogenic callus cultures contain differentiated embryogenically competent cells that regenerate complete plants. Non-embryogenic calli contain homogenous, dedifferentiated cells, which are used for secondary metabolite production. Suspension cell cultures are frequently used for mass cultivation in specially designed bioreactors.
There are remarkable similarities between the embryogenesis of calli and the gall crown tumor formation of plants. Therefore, the molecular regulatory process in plant tumors is partially comparable to those in callus cultures. During tumorigenesis, the bacterial genome is inserted into the host genome; this activates the normal pathways of phytohormone accumulation and alters the plant’s cellular response to phytohormones. Either the phytohormones bind to their cellular receptors, leading to activated expression of downstream genes, or else T-DNAs stimulate plant cell growth, even in the absence of phytohormones [29].
4. Production of secondary metabolites for therapeutic purposes
4.1. Bioactive phytochemicals
A number of applications of callus cultures have commercial potential, four of which will be discussed here in more detail: ① the production of secondary metabolites for therapeutic purposes, ② the production of therapeutic antibodies and other recombinant proteins, ③ the production of agricultural plants by regeneration from calli, and ④ the production of horticultural plants by the same means.
Callus cultures may be used for the sustainable and large-scale production of secondary metabolites in pharmaceuticals, cosmetic food, and related industries. Callus cultures from medicinal plants produce bioactive phytochemicals that can be used to treat a wide variety of diseases (e.g., cancer, cardiovascular diseases, neurodegenerative diseases, infectious diseases, etc.); furthermore, the produced chemical substances do not seem to be limited to certain chemical classes, but have a wide chemical variety (Table 1) [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78]. An example is given in Fig. 2 [67]. As phytochemicals can be directly extracted from calli without sacrificing the entire plant, the callus technology may help to protect rare and endangered plant species, and sufficient amounts of secondary metabolites can be produced in vitro. Callus cultures can also be converted to single-cell suspension cultures growing in flasks on shakers or in biofermentors in order to produce the desired secondary metabolites [79]. This allows growth under controlled conditions without the influence of varying environmental factors, seasonal variation microbial diseases, pests, and geographical constraints. Hence, secondary metabolites with constantly high quality can be produced.
Table 1. Generation of phytochemicals by callus cultures derived from selected medicinal plants.
| Name | Phytochemical produced | Reference |
|---|---|---|
| Abrus pecatorius | Glycyrrhizin | [30] |
| Ammi majus | Furocoumarine | [31] |
| Arachis hypogaea | Resveratrol | [32] |
| Aralia elata | Aralin | [33] |
| Arnebia euchroma | Shikonin | [34] |
| Brugmansia candida | Hyoscyamine | [35] |
| Brugmansia suaveolens | Tropane alkaloids | [36] |
| Calendula officialis | Oleanolic acid | [37] |
| Calypogeia granulata | 1,4-dimethylazulene | [38] |
| Camptotheca acuminata | Camptothecin and 10-hydroxycamptothecin | [39] |
| Carthamus tinctorius | α-tocopherol | [40] |
| Chrysanthemum cinerariefolium | Quercetin | [41] |
| Coleus blumei | Rosmarinic acid | [42] |
| Datura stramonium | Tropane alkaloids | [43] |
| Drosera burmanii | Plumbagin | [44] |
| Ephedra (E.) andina, E. distachya, E. equisitina, E. fragilis var. camplyopoda, E. gerardiana, E. intermedia, E. major ssp. procera, E. minima, and E. saxatilis | 1-ephedrine and d-pseudoephedrine | [45] |
| Eschscholtzia california | Sanguinarine | [46] |
| Ginkgo biloba L. | Ginkgolide B and ginsenoside | [47], [48], [49], [50] |
| Glycine max | Apigenin, daidzein, genistein, and luteolin | [51] |
| Glycyrrhiza glabra | Soyasaponin | [52] |
| Hypericum perforatum | Hypericinn and hyperforin | [53] |
| Hypericum perforatum L. | Hypericin | [54] |
| Linum flavum | Podophyllotoxin | [32] |
| Linum narbonense | Justicidin B | [55] |
| Linumtenuifolium, L. bienne (L.) tenuifolium, L. bienne,and L. glaucum | Justicidin B | [56] |
| Lithospermum erythrorhizon | Shikonin and rosmarinic acid | [57], [58] |
| Lychnophora ericoides | Furanoheliangolide | [59] |
| Lycopersicon esculentum | Scopoletin | [46] |
| Ocimum basilicum | Rosmarinic acid | [60] |
| Panax ginseng | Saponins | [61] |
| Pharbitis nil | Umbelliferone and scopoletin | [62] |
| Podophyllum hexandrum | Podophyllotoxin | [32] |
| Rauwolfia serpentina | Ajmaline, serpentine and reserpine | [63] |
| Rhodolia sachalinensi | Salidrosides | [64] |
| Rubia tinctorum | Tropane alkaloids | [65] |
| Ruta (R.) bracteosa, R. chalepensis, and R. macrophylla | Acridone and furoquinoline alkaloids and coumarins | [66] |
| Salvia miltiorrhiza | Rosmarinic acid, salvianolic acid B, and diterpenoid tanshinones | [67], [68], [69], [70] |
| Saussurea medusa | Flavonoids | [71] |
| Scopalia parviflora | Scopolamine | [72] |
| Silybium marianum | Silymarin | [73] |
| Solidago chilensis | Chlorogenic acid and rutin | [74] |
| Taxus baccata | Paclitaxel | [75] |
| Taxus chinensis | Paclitaxel | [76], [77] |
| Vitis vinifera | Stilbene, resveratrol, and anthocyanins | [32], [78] |
 Fig. 2. Bioactivity testing of callus cultures derived from Salvia miltiorrhiza. (a) Callus cultures from leaf and stem; (b) high-pressure liquid chromatography (HPLC) chromatograms of rosmarinic acid (RA) and salvianolic acid (Sal B) from the plant stem and stem-derived callus culture, where HPLC profiles of the plant leaves and leaf-derived callus cultures revealed similar results (data not shown); (c) chemical structures of rosmarinic acid and salvianolic acid; (d) cytotoxicity of steam callus extract, leaf callus extract, isolated RA, and isolated Sal B toward CCRF-CEM leukemia cells as determined by resazurin reduction assays. DMSO: dimethylsulfoxide. Reproduced from Ref. [67] with permission of Elsevier Ltd., ©2016.
Fig. 2. Bioactivity testing of callus cultures derived from Salvia miltiorrhiza. (a) Callus cultures from leaf and stem; (b) high-pressure liquid chromatography (HPLC) chromatograms of rosmarinic acid (RA) and salvianolic acid (Sal B) from the plant stem and stem-derived callus culture, where HPLC profiles of the plant leaves and leaf-derived callus cultures revealed similar results (data not shown); (c) chemical structures of rosmarinic acid and salvianolic acid; (d) cytotoxicity of steam callus extract, leaf callus extract, isolated RA, and isolated Sal B toward CCRF-CEM leukemia cells as determined by resazurin reduction assays. DMSO: dimethylsulfoxide. Reproduced from Ref. [67] with permission of Elsevier Ltd., ©2016.