. Introduction
The knee is the articulating joint most commonly affected by osteoarthritis [1], and there are still major challenges to overcome in the development of lasting treatments. There is now an increasing effort to develop early stage interventions to prevent knee degeneration and delay the need for joint replacement surgery. This includes regenerative therapies for cartilage and bone [2], as well as repairs for the meniscus [3] and ligaments [4]. Development of such tissue-sparing interventions requires an understanding of the mechanical environment of the knee, necessitating improved pre-clinical testing methods such as in vitro simulation [5]. Experimental methods can provide a controlled environment for assessing joint mechanics, but are generally expensive and time-intensive when using large numbers of in vitro specimens or in vivo subjects, and are limited in the scenarios and outputs that can practically be investigated. Computational models therefore play an important role in non-invasively understanding knee mechanics [6]; they can provide information that would be difficult or impossible to obtain from experimental studies and can also be utilised for sensitivity testing in order to assist the setup of experimental models.
Finite element (FE) modelling has been used extensively in biomechanics, and a growing number of studies of the knee that use FE methods are being reported. Examples include the investigation of cartilage degeneration and osteochondral defects [7], [8], [9], the influence of meniscus shape [10], [11] and the biphasic response of cartilage to loading [12]. Models have also been developed to investigate stresses in the patellofemoral joint [13], which is beyond the scope of the present review.
The computational investigation of the contact mechanics of the tibiofemoral joint is particularly challenging because there are multiple contacts between tissues and complex articulating surfaces. Validation of knee models is therefore non-trivial, and despite the large body of work, there has so far been only limited progress in translating the findings and tools of modelling research into clinical practice. This may become a more common aim as modelling technology progresses. Because of the complexities in terms of the structures, material representations and forces that can be included in knee models, many studies currently aim for increased understanding of the knee's mechanical behaviour, particularly in the context of disease scenarios where interventions are becoming increasingly common. These investigations may occur on highly subject-specific models or more generic representations of the knee.
Kazemi et al. [6] wrote an extensive review on advances in computational mechanics in the human knee in 2013, and a more general review on knee biomechanics was provided by Madeti et al. [14] in 2015, but there has since been significant further work produced, especially in the area of model validation. The purpose of the present review is to provide an overview of the main processes and current challenges in knee modelling, and then to focus on examining validation strategies and the circumstances in which validation can be omitted. Three particular categories of study are identified: those representing trends and sensitivities, often using generic models; and two types of more subject-specific models, representing in vitro specimens and representing in vivo subjects. This review is not intended to provide a comprehensive list of papers utilising FE models to investigate the knee, but to focus on the key challenges and the state of the art for validation when these different study types are utilised. This includes highlighting the importance of model reuse, verification, calibration and context of use, as well as discussing good practices and potential areas for future development.
2. Processes and challenges for knee models
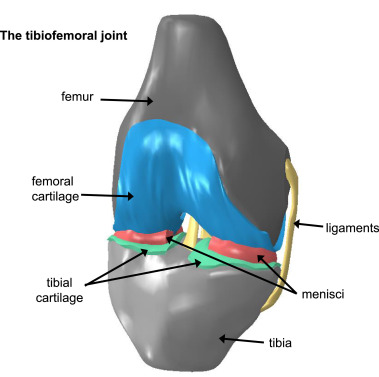
The knee is a highly complex physical system and comprehensive models remain elusive due to the sparsity of precise data on knee tissue properties and limited understanding of the interactions between them, as well as how these factors vary among different subjects. Therefore models of the knee may be generated by considering a subset of the system, pragmatically chosen based on a focused question that the model is designed to address. The focus of studies modelling the tibiofemoral joint in particular is often on the behaviour of the cartilage, meniscus or ligaments (Fig. 1).
 Fig. 1. Diagram of some of the typical components commonly featured in computational models of the tibiofemoral joint.
Fig. 1. Diagram of some of the typical components commonly featured in computational models of the tibiofemoral joint.In addition to validation, which will be covered in detail in Section 3, there are several key challenges to address in order to develop computational models of the tibiofemoral joint. These include:
-
(1)
The capture and representation of appropriate geometry and material properties.
-
(2)
The representation of appropriate motions, loads and constraints.
-
(3)
The establishment of relevant outputs and their levels of uncertainty.
2.1. Inclusion of geometry and material properties
2.1.1. Geometry
The level of detail required for the representation of the structures in the knee depends on the particular application of the model. For example, in a study focussed primarily on cartilage response, it may be appropriate to use basic spring elements to represent ligaments, or even omit them completely for computational efficiency in which case their effect on the primary tissue of interest should be taken into account using kinematic constraints [7]. Thus there exists variation among models in the manner of implementation and detail in the representation of different structures in the knee. There are several options for including constituent tissues (e.g. ligaments, meniscus), in knee models:
-
•
The tissues can be explicitly modelled, and output measures taken (with detail driven by output precision/accuracy).
-
•
The tissues can be explicitly modelled, but perform a supporting role in the model (with detail driven by precision/accuracy of their effect on the outputs of interest).
-
•
The effect of the tissues can be wrapped into boundary conditions and loads, with no geometry included (applies primarily to ligaments).
For tissues that are included with explicit geometric representation, geometry is usually incorporated by utilising medical imaging (CT or MRI) of cadaveric specimens, volunteers or patients [15], [16], [17]. Dependent on image resolution, this can provide an approximation of the native joint geometry, although it does not provide a true representation since there will be errors due to image resolution, imaging artefacts and simplifications inherent in the segmentation process [18], as well as smoothing applied to specimen-specific models to ensure robustness of contact algorithm solutions [19]. In some cases, multiple users may perform segmentation to minimise variability [15], with variation between paired images required to be below a specified threshold [20].
Pena and colleagues provided some earlier instances of models using CT and MR imaging to include all of the main structures of the knee, producing models featuring cartilage layers, menisci and ligaments, as well as rigid bone representations [21], [22]. These models were used to analyse the effects of meniscectomy [22], and later to investigate the combined role of menisci and ligaments in load transmission and knee joint stability [21]. Although only basic validation was provided in terms of comparisons to the kinematics and stresses reported by other studies using different subjects, and an idealised model was necessary to test mesh convergence, these studies nevertheless demonstrated the potential for subject-specific FE models to predict complex stress and strain patterns and kinematics occurring in knee joints.
As an alternative to segmentation-based models, the geometry for knee models can also be described mathematically [23], [24], [25], reducing computation and analysis time [26], [27], [28]. It has been shown recently that trends predicted by idealised parametric models of joints based on mathematical geometric descriptors can be similar to those seen in models based on image segmentation, in both the knee and hip joints [10], [29], [30]. Thus simplified models can provide reliable qualitative predictions of expected trends, with particular potential to identify the aspects on which to focus in more sophisticated models. However, quantitative data from idealised models may not match well with experimental predictions [10], [31]. When a fully parametric geometric approach is taken, levels of geometric complexity can be added depending on the intended application. For example, the menisci play an important role in knee stability and they are essential for both load transmission and joint lubrication [32]. Meniscus injury is associated with osteoarthritis [33], and meniscal pathology provides a key biomarker for osteoarthritis progression. Modelling the behaviour of the menisci can therefore potentially provide new insight into disease progression and a parametric approach can be taken to investigate the effects of different meniscal geometries [11]. On the other hand, a study focused on forces occurring in osteochondral grafts during cartilage-on-cartilage contact may include a more basic meniscal representation [8].
2.1.2. Material properties
In terms of material properties, a large amount is known about the internal structure and properties of the bone, articular cartilage, meniscus, and ligaments within the knee. Equally, there exist theoretical models and numerical techniques that include collagen fibre alignment, hyperelastic behaviour, fluid contribution and multi-layered aspects of the knee as a mechanical system [12], [15], [34], [35]. However, obtaining sufficiently detailed experimental data to calculate the many property values required for such sophisticated representations is very challenging. Thus the material property models within computational knee simulations are commonly set up based on a sensible choice of physics informed by literature and not explicitly validated. There is great variability in the properties used for each tissue, which may be taken from existing literature or be derived experimentally. Imaging can also be used to obtain subject-specific material properties; for example location-specific bone density is commonly derived using CT imaging [36], [37], [38], and one study [39] used sodium MRI to determine fixed charge density distributions in the tibial cartilage, although this technology is limited by imaging resolution.
Bone is often assumed to be rigid in knee models for comparative studies where loading effects on cartilaginous soft tissues or ligaments are of particular interest [10], [16]. A more complex bone representation may be important for making subject-specific predictions of regions at risk of joint failure for particular specimens, as bone stiffness can affect tibial cartilage stresses [36].
For representing cartilage, a linear elastic material model is commonly used, due to the equivalence between short-time biphasic and incompressible elastic material responses demonstrated by Ateshian et al. [40]. Depth-dependent material properties, inhomogeneity of the cartilage and the biphasic response may also be relevant if the intended application is to better understand longer-term cartilage mechanics [12], [34], [41]. Osteochondral defects, consisting of damage to both articular cartilage and the underlying subchondral bone, present a large clinical burden by altering the local biomechanics and biotribology of the knee joint and causing joint pain [2]. Whilst imaging approaches can be used to assess cartilage deformation [42], such methods do not provide an effective means to analyse the contact force distribution on the articulating surfaces. Inclusion of detailed cartilage layers within FE models of the knee is therefore crucial for progressing understanding of their degeneration. This may include multiscale modelling of cartilage [43], [44], [45]. Freutel et al. [46] discussed the challenges of material models for soft tissues in greater detail, so this will not be covered further here.
2.2. Inclusion of motions and loads
This section is concerned with the challenge of ensuring knee models produce motions corresponding to an experimental situation of interest. This requires an understanding of the moving parts within the knee and how they react to different motions modifying the mechanical environment. Both computationally and experimentally, motions may be applied directly or they may result from applied loads when translational or rotational freedoms are applied to the femur or tibia. The application of only loads without any constraints on motion allows the model the most freedom but could result in physiologically inaccurate movements. Different modelling studies therefore approximate knee function in different ways. Loads may be controlled and adjusted for sensitivity purposes [11], or specific loads and boundary conditions may be chosen to ensure motions replicate experiments [47].
Specific in vivo motions can be difficult to derive; Stentz et al. [48] reported using CT bone models combined with a dynamic radiostereometric analysissystem to achieve non-invasive measurements of joint kinematics. This approach to measuring knee movement was validated against a gold standard skin marker method, and has potential clinical applications to prosthesis migration. Deriving in vivo knee forces for use in quasi-static FE models may also be achieved using multibody models [49], [50]. Fully dynamic FE models may be necessary if the effects of inertia were thought to be crucial, for example in a study of knee replacements [49].
Ligaments play a large role in both knee kinematics and biomechanical load bearing [51] and their injury is associated with increases in pain, osteoarthritis and knee joint instability [33], [52]. Ligaments are therefore a key factor which influence the relationship between applied loads (or motions) and resulting motions (or loads) in the knee. One of the principal clinical drivers for the detailed modelling of ligaments in the knee is the analysis of their repair after injury, particularly the anterior and posterior cruciate ligaments (ACL and PCL), and FE models are increasingly being utilised to investigate ligament rupture and reconstruction [53], [54], [55]. Ali et al. [16] demonstrated that ACLresection can produce altered knee mechanics and motion by testing cadaveric knee specimens in an electro-hydraulic knee simulator with motor-actuated quadriceps and loads applied at the hip and ankle, in each case first with the ligament intact and then resected. FE models developed to simulate these scenarios revealed that changes resulting from ACL resection can manifest differently among different specimens; one specimen exhibited altered anterior tibial translation, whilst the other exhibited elevated joint loads. Since individual differences exhibited most clearly when calibrated ligament properties were used, this suggests a subject-specific ligament modelling approach would be beneficial for a larger study. This is supported by findings of Beidokhti et al. [15], who found that including subject-specific derived ligament properties in continuum modelled ligaments improved predictions of experimental kinematics and contact pressures. Earlier models [19] also found that tuning ligament properties so that model kinematics matched those found in a cadaveric specimen aided the validation of model derived joint contact forces. More recently, it has been suggested that additional peripheral soft tissues including knee capsules as well as ligaments may alter predicted knee mechanics [56].
Another major challenge related to modelling knee motion is understanding the role of the meniscus and analysing meniscal movement in response to loading. This may be crucial for modelling the potential for damage progression in the knee. Meniscal translation has previously been captured using MR imaging [57], and Halonen et al. [47] created a subject-specific FE model using MR images of a volunteer's knee specifically to investigate meniscus movements and cartilage strains. One particular issue with accurately modelling the meniscus is incorporating meniscal attachments. This aspect can be difficult to accurately capture within models due to the challenge of achieving precise segmentation of attachment site geometry and establishing material models for their behaviour. The attachment sites can be particularly difficult to identify in imaging, especially without prior ligament removal if using a cadaveric specimen, and may be virtually impossible to distinguish from other soft tissues if using CT imaging. Thus generic spring elements with estimated properties and locations have been used to represent meniscal attachments, which along with friction serve to limit meniscal movement. Freutel et al. [58] segmented medial meniscus geometry from MR images of porcine knee joints, with meniscal displacements having previously been determined experimentally. From this data, optimisation was used to determine subject-specific material properties of the meniscus and its attachments, allowing the time-dependent behaviour of the meniscus and its attachments to be investigated.
2.3. Input precision and establishing relevant outputs
There are many different motivations for developing computational models of the knee, producing many distinctive approaches to doing so. It is therefore important to consider the relevance of model outputs assessed to the original aim of the study. In particular, when the ultimate aim is to use the models to predict disease risk or assess the suitability of treatments in vivo, it is necessary to consider the clinical relevance of outputs reported [59]. This may include outputs to indicate risk of damage progression or to assess intervention suitability. For example, meniscus movement might be a crucial metric in a study of the progression of meniscal tears [58], whilst in a study of femoral osteochondral grafts it may be pertinent to analyse tibial cartilage contact patterns to understand the effects of graft recession or extrusion [8], [60].
In the case of FE models used to examine mechanical response in the knee, model outputs can be highly sensitive to the chosen representation and condition of included tissues such as the menisci and cartilage. Ambiguity in input values can result in a wide range of reported values for outputs of interest. One study [61] found that output uncertainty can be reduced when specimen-specific data for certain input parameters is known, including joint geometry such as meniscal insertion site positions, kinematics and BMI to inform loading. Thus some uncertainty can be reduced for specimen-specific models, although many inputs may be difficult or impossible to obtain clinically. Furthermore, in certain situations some parameters may be impossible to control experimentally, and in this case they may be used as tuning parameters for each knee-specific model. For example, an earlier study by the same group [62] used the varus-valgus angle as a tuning parameter and found similar regions of contact stress between models and experimental work (in this case quantified by normalised cross correlation values within 69 to 85%).
Even when the uncertainty for output measures is minimised, it remains crucial to establish exactly which outputs are of interest for the particular focus of the model so that they can be used to predict intervention response or disease progression. The Osteoarthritis Initiative (OAI) [63] provides a database on the natural history of osteoarthritis by making publically available clinical evaluation data and imaging (X-ray and MRI) for nearly 5000 subjects. One study [7] considering subjects from the OAI database defined outputs specific to disease progression by splitting subjects into two groups based on osteoarthritis risk and BMI. FE models of one representative subject from each group were generated and collagen fibril damage was defined to occur when tensile stresses exceeded a threshold limit during gait loading, with control of degeneration based on the duration of loading in different regions over successive iterations. In this way an algorithm was presented to predict knee cartilage degeneration based on accumulated excessive stresses in the medial tibiofemoral compartment. Approaches like this may become more common as modelling complexity increases, allowing outputs relevant to specific scenarios to be analysed.
2.4. Model sharing
As the prominence of computational modelling in biomechanics increases, uncertainty about modelling results can be decreased through increased sharing of models and data [64], [65]. It can be a challenge to fully describe the methods used in FE models of the knee within research papers, but this is important for understanding simplifications that may affect the results. Sharing models and data of sensitivity studies in particular can help clarify the effects of different levels of input precision on likely model outcomes. Sharing of scripts and protocols in addition to data can help mitigate potential issues with software and version compatibility and ensure repeatability. In addition to the OAI project [63] mentioned previously, other researchers are beginning to make knee models freely available online [16], [66], [67]. This can have significant impact; models made available through the Open Knee Project [66] have supported many new publications, including [10], [11], [12], [68]. Sharing of models also provides the means for improved understanding of what aspects of the model were validated. Transparently reporting numerical quantification of validation evidence is also essential, because it is plausible that a given set of experimental and computational results would be described as similar by one group where another would conclude that the model has failed to precisely replicate the experiment. Knee model validation is the focus of the next section.
3. Knee model validation and calibration strategies
3.1. Validation, verification and calibration
Having considered some of the ways in which methodological challenges in knee modelling are being addressed, the challenge of providing validation for computational models of the knee is now examined. To validate a model is to provide evidence that model generated results correspond to the outcomes of the real world scenario simulated [69]. Several guidelines exist with considerations for reporting FE validation studies in biomechanics and including sufficient detail for repeatability [70], [71], [72]. In particular, Pathmanathan et al. [73] recently proposed a framework for the applicability analysis of validation evidence in computational models for biomedical applications, and this provides a resource for evaluating validation quality. The framework recommends the systematic assessment of the relevance of the validation evidence for the proposed context of use, which encompasses the purpose of the model and what factors its results are used to inform.
For the purposes of this paper, direct validation is used to refer to situations in which a comparison between a model prediction and an experimental test result is made after a model has been developed to match the corresponding experiment as closely as possible [69]. Indirect validation is used when the model prediction is compared to a physical case where it not known whether the conditions are the same. Confidence can also be built by performing several related validation checks, for example by comparing:
-
•
Several different outputs (e.g. displacement, stress)
-
•
Under a variety of conditions (e.g. loading cases, restraint cases)
-
•
Against data sets from a variety of sources (e.g. multiple specimens)
Prior to validation of a model, it is necessary to assess the ability of the model to provide accurate numerical approximations to its underlying equations, including the testing of mesh convergence. This is known as model verification, which has been widely discussed [69], [70] and will not be covered in detail here. However it is important to highlight that solving computational contact problems in biomechanics generally remains very challenging, and image processing and smoothing steps may be necessary to achieve model convergence. Contact algorithms in finite element codes may not be sufficiently robust to handle meshes on complex specimen-specific geometries such as those found in the knee, with small changes to geometry resulting in models that do not converge. Through the FEBio project [74], open-source code has been specifically designed for such biomechanical applications and is addressing some of these challenges.
One aspect of verification for which the modeller is responsible is demonstrating the suitability of the chosen mesh. Hexahedral elements are generally preferred for modelling contact, but present a particular challenge when meshing complex geometries such as femoral cartilage and the menisci. Quadratic tetrahedral elements provide a possible alternative to alleviate this issue; they are more straightforward to implement and have previously been seen to perform well in models of foot biomechanics [75] and articular contact in the hip [76], [77]. Recently some authors have also used quadratic tetrahedral elements in modelling knee joints [15].
Computational models can be developed contemporaneously with, and validated against, in vitro or in vivo experiments. This may require some calibration of model parameters so that model results align with experimental results [78]. Calibration involves tuning input parameters based on model results. If these tuned parameters are not specific to each specimen, this generally means minimising the model-experiment error across a set of specimens. A gold standard for validation is thus to test whether model results continue to correspond well with experimental data when independent specimens are tested. However, because several factors affect their outputs, it is important to avoid erroneously concluding a model is validated based on its calibration. At the study design phase, researchers should carefully consider what their models aim to elucidate and plan validation steps accordingly. Several different combinations of model parameters may lead to similar results, and consequently it may be possible to erroneously ‘validate’ a model by chance. Parameters that initially appear unimportant could cause crucial differences in model output following the addition of further parameters. For example, in a model of knee contact mechanics, calibrating the meniscus properties may produce the cartilage contact pressures that align well with experimental data, but the meniscus properties themselves may actually be incorrect. These incorrect properties, coupled with incorrect properties of meniscal attachments and the coefficient of friction between the meniscus and cartilage layers could lead to inaccurate conclusions about meniscal pathology even if cartilage pressures were observed to be correct. Experimental data also has limitations (for example in resolution and accuracy of sensors and detection of environmental noise), so researchers should take into consideration that model outputs may need to be compared to suboptimal experimental data. There is substantial literature on sensitivity testing in knee models [10], [61]and researchers should be encouraged to report these findings to provide the community with an improved basis for output interpretation and understanding of the circumstances in which conclusions remain valid.
3.2. Approaches to validation in different study designs
Validation is often more challenging when models are developed to represent in vivo subjects. On the other hand, generic models aiming for broad conclusions may not require detailed validation strategies. Calibration and validation strategies used in different knee modelling studies therefore vary according to the study purpose and the types of model utilised; this is summarised in Table 1.
Table 1. Summary of the main types of model used in finite element studies of the knee. Here subject-specificity is used to refer to the geometric representation employed in the model, but calibration of material properties may mean that the materials could also be described as subject-specific.
| Model type | Specificity | Calibration | Validation | Possible Uses |
|---|---|---|---|---|
| Representing trends and sensitivities | Generic or subject-specific | Not included | May report literature comparisons | Independent demonstration of the effect of inputs and interactions within a complex physical system. May also be used complementary to experiments to reveal internal stresses and strains or provide sensitivity data. |
| Representing in vitro subjects | Subject-specific | Frequently included | Matching experiment | Testing a device, procedure or disease in the context of particular in vitro specimens. |
| Representing in vivo subjects | Subject-specific | Challenging to include | Matching in vivo data | Testing a device, procedure or disease in in the context of particular in vivo subjects. |
Further discussion of each of these identified approaches is provided in subsequent parts of this section. Some of the key studies discussed are outlined in Table 2 to demonstrate examples of each study type from the literature.
Table 2. Applications of a selection of recent finite element modelling studies of the human tibiofemoral joint, arranged into the three identified study types to highlight the validation strategies used. Model parameters such as material properties are commonly taken from literature, so instances of calibration are also highlighted.