1. Introduction
As a result of the industrial revolution, humanity has become dependent on fossil fuels. The continued use of fossil fuels produces significant amounts of air pollution, notably CO2 [1]. Nations all across the world have set a target in recent years to reduce greenhouse gas emissions, which have been related to global warming [2]. The role of sustainable and renewable energy in reducing and eliminating emissions is becoming increasingly important. Fuel Cells (FC) are one of the most promising energy conversion and storage technologies on the market today. Scientists have conducted several tests to assess and improve the performance of fuel cells over the last several decades.
There are regions on the planet where a large portion of the population does not have access to electricity [3]. Clean and easily accessible renewable energy is urgently needed not only to address climate change challenges [4], but also to give more people who have never had access to power the opportunity to do so. Renewable energy, such as hydrogen-powered fuel cells, has been included in the energy plans of various governments. South Korea, for example, intends to broadly use hydrogen-fueled fuel cells by 2050 [5,6]. Other forms of fuel cells, such as microbial fuel cells and methanol fuel cells, have a lot of potential as long-term and feasible energy-generating systems [4,7].
Fuel cells use electrochemical reactions rather than combustion to generate power from many types of fuels [8,9]. Fuel cells utilise a variety of fuels, including renewable and ecologically acceptable fuels, and convert their chemical energy into electrical energy more efficiently than other energy conversion systems [10]. When compared to diesel engines, fuel cells can be as efficient as 90% in terms of energy conversion efficiency [11]. Fuel cells are not only efficient, but they also have little to no environmental impact. They are quiet due to the lack of moving parts, are easy to scale, and are more portable than conventional energy conversion devices [[10], [11], [12]] Fuel cells run on renewable resources, reducing reliance on fossil fuels. These fuels are fairly diverse and can be tailored to the abundance of resources available in the area of operations. Despite their high upfront costs, fuel cells have low operational expenses [11].
The two electrodes (anode and cathode) and the electrolyte are the most important components of fuel cells. The anode receives the fuel, whereas the cathode receives the oxygen. The anode's fuel is oxidized, and electrons are released. Electrons go from the anode to the cathode via the external circuit, producing electrical current. The electrolyte can only be transmitted by ions, not electrons. Electricity is produced when electrons flow through an external circuit [13]. In fuel cells, the electrochemical interaction between fuel and oxygen produces electrical energy, H2O, CO2, and some waste heat, which is significantly less than that produced by conventional combustion engines [14].
Fuel cells are classified into several categories based on their working circumstances, electrolyte composition, and fuel type. The proton exchange membrane fuel cell is a form of fuel cell that demonstrates the aforementioned benefits (“PEMFC”). This type of fuel cell has a high energy density and is fairly tiny in size. They also employ hydrogen as a fuel, resulting in an environmentally favorable flue gas and a renewable fuel. However, there are a few difficulties with PEMFC [7].
The first problem is that a low working temperature is required. PEMFC suffers from dehydration at high temperatures when utilizing Nafion membranes, resulting in a loss in performance efficiency. Research on membranes capable of working at high temperatures is ongoing, with options such as benzimidazolesemployed as the polymer base [15]. Different polymers and additional applications for high temperature fuel cell membranes are also being investigated [16]. The ability to run fuel cells at greater temperatures has other advantages, such as the ability to use purer hydrogen as fuel for increased efficiency. This increase is due to the fact that the heat optimally required for H2desorption is slightly above 100 °C, which cannot be accommodated by a PEMfuel cell operating at lower temperatures [17].
Water and energy management improve when PEMFCs are run at high temperatures. Good energy management systems can be crucial in PEMFC applications such as fuel cell hybrid electric vehicles [18]. Because of the low operating temperatures, establishing good thermal and water management in Fuel Cell Hybrid Electric Vehicle (FCHEV) may be problematic [19]. Methods such as utilizing an electro spun micro-porous layer for improved water management in PEMFC systems have been researched [20]. Water management is simpler since the water produced by high temperature fuel cells exists totally as vapor. A higher temperature also suggests that fuel cell systems can withstand a higher level of CO poisoning [21].
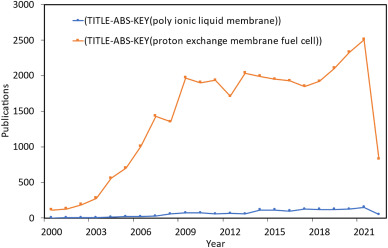
Another significant concern is the high capital cost of manufacturing PEMFC. PEMFCs generally use platinum as a catalyst and Nafion as a membrane, both of which are fairly expensive even when compared to other forms of fuel cells. A study to design a new PEM to replace Nafion would be a step forward in the development of PEMFC [22]. One of the most commonly utilized membranes for electrochemical energy device applications is Nafion-based proton conducting membranes [23,24]. Because of their high thermal and mechanical durability, polyionic liquids have received interest as proton exchange membrane materials [25]. PIL membrane utilization, particularly for fuel cells, has the potential to outperform even conventional energy generation technologies [26]. Further study is being performed on a number of promising compounds, including diethylmethylammonium trifluoromethanesulfonate and Nafion/PCIL combinations [27,28]. Data for the total research that has been done on the topic based on chosen keywords as search input in Scopus database is shown in Fig. 1.
 Fig. 1. Literature published on Scopus for “Polyionic liquid membrane” and “proton exchange membrane fuel cell” by year since 2000.
Fig. 1. Literature published on Scopus for “Polyionic liquid membrane” and “proton exchange membrane fuel cell” by year since 2000.2. Fuel cell
2.1. Proton exchange membrane fuel cell
The membrane is a critical component in PEMFC, as it ultimately determines the performance of the fuel cell. PEMFCs are currently most typically used with Nafion-based membranes [16]. Proton exchange membranes employ hydrogen as fuel and primarily function at temperatures below 250 °C, classifying them as low temperature fuel cells [29]. PEMFCs use polymer blends as an ion exchange membrane as an electrolyte to conduct protons, as shown in Fig. 2a [11]. Hydrogen fuel production technologies for PEMFC are being studied, such as decarbonizing fossil fuels [17] (see Fig. 3).
 Fig. 2. (a) - Mechanism diagram of a PEMFC; (b) - Schematic Diagram of a Direct Methanol Fuel Cell; (c) - Schematic Diagram of a Molten Carbonate fuel cell; (d) - Schematic Diagram of a Solid Oxide Fuel Cell.
Fig. 2. (a) - Mechanism diagram of a PEMFC; (b) - Schematic Diagram of a Direct Methanol Fuel Cell; (c) - Schematic Diagram of a Molten Carbonate fuel cell; (d) - Schematic Diagram of a Solid Oxide Fuel Cell. Fig. 3. (a) List of IL cations and anions and (b) Structure of common IL cations and anions.
Fig. 3. (a) List of IL cations and anions and (b) Structure of common IL cations and anions.Because of their variable power range, PEMFCs have a wide range of applications [30]. PEMFC-powered vehicles have been developed in an effort to lessen the environmental impact of hazardous emissions and air pollution [31]. Because of their compact size and quiet operation, PEMFC are also excellent for household power generation. However, mass commercialization of PEM fuel cell vehicles remains a difficulty due to their low working temperature, high cost, and low durability [32].
PILs and protic ILs are being investigated for use as proton exchange membranes in PEMFCs. The effect of hydrophobic and hydrophilic PILs on the characteristics of PVDF membranes has yielded some promising results in terms of degradation temperature and proton conductivity [[33], [34]] [[,][34]]. Proton conducting benzamidazole/PIL mixtures show great promise for high temperature PEMFC membranes [35]. The majority of PEM fuel cell membrane research is aimed at boosting efficiency at higher temperatures (>100 °C) [36,37].
Operating a PEMFC at high temperatures demands some additional requirements. Running PEMFCs with Pt catalysts under high conditions, such as temperatures exceeding 80 °C, may result in agglomeration and degradation of the Pt catalyst [38]. At higher temperatures, dehydration of the proton exchange membrane reduces efficiency [39]. This means that the PEM does not have to rely as heavily on water to achieve its high proton conductivity. Other materials will also need to be modified based on the material of the membrane (i.e. zeolite and graphene based membrane) [[40], [41], [42], [43], [44], [45], [46]] [[40], [41], [42], [43], [44], [45], [46]] [[40], [41], [42], [43], [44], [45], [46]]. PEMFC has the potential energy generation orders of magnitude ahead of other types of fuel cells [47].
The electrochemical reaction in the PEMFC uses H2 and O2 gases. The reaction happens in the Membrane Electrode Assembly (MEA), which consists of the gas diffusion layer, catalyst layer, bipolar plate, and the proton exchange membrane (PEM) [48]. The H2 gas in the anode will flow through the gas diffusion layer and reach the catalyst layer, where the individual H2 will be dissociated into two electrons and two protons. Both of the protons will pass through the PEMs to reach the cathode's catalyst layer, where the electrons will pass through an external circuit as an electrical current. Then, the air in the cathode will react with the transferred electrons and protons into heat and H2O [49]. The reaction in the anode and cathode can be seen in Eq. (1) and Eq. (2).
Anodic:(1)
Cathodic:(2)
The overall reactions in PEMFC can be seen on Eq. (3) [32].(3)
2.2. Direct methanol fuel cell
In the anode of a direct methanol fuel cell (“DMFC”), methanol and water react to form hydrogen ions, electrons, and carbon dioxide. The hydrogen ions flow through the electrolyte while the electrons flow through the external circuit. In the cathode, electrons, hydrogen ions, and oxygen react to form water. The flow of electrons in the external circuit creates electricity. The mechanism diagram of DMFC can be seen on Fig. 2b.
Just like PEMFC, DMFC suffers from low operating temperatures and would have better performance at a high temperature. High operating temperatures would improve the tolerance of catalysts towards carbon monoxide and allow easier cooling systems [50]. Another disadvantage of DMFC is the low electrocatalytic activity of methanol and oxygen reactions [51]. It is estimated that DMFC will be a viable option for energy generation when conditions such as 100 W/l power density, 1000 W/hl energy density, a durability of at least 5000 h, and a cost of at most $3/W [52]. This is a challenge as the catalyst used in DMFC is made of platinum, which is quite expensive and difficult for mass commercialization. This is the reason for research and attempted developments in new catalysts for not only DMFC but also for PEMFC, which is for new and more accessible non–Pt catalysts [53].
Other DMFC research focuses on developing a novel membrane that can endure higher temperatures during operation. Membranes with low methanol crossover and the ability to operate at high temperatures were investigated. SPEEK, or sulfonated poly(ether, ether ketone) membranes, are one type of material used in this study [54]. Other studies are looking for better support materials for DMFC electro catalysis [55].
2.3. Molten carbonate fuel cell
The carbonate ion is generated when carbon dioxide, oxygen, and electrons react in the molten carbonate fuel cell (“MCFC”). An electrolyte transports the carbonate ion to the anode. In the anode, the carbonate ion interacts with hydrogen to form water, carbon dioxide, and electrons. Electrons from the anode go through an external circuit to the cathode, where they combine to produce electricity [56]. The mechanism diagram of MCFC can be seen on Fig. 2c.
There are significant distinctions between MCFC and PEMFC. To begin with, the electrode utilized in MCFC is a relatively inexpensive material, nickel. This is far less expensive than the platinum catalyst used in PEMFC [57]. To function efficiently, MCFC must also be run at a high temperature, around 600–650 °C, to prepare the anode by Ni oxidation. At said temperature, it was found through TGA that a slow weight gain was observed due to oxidation of the Ni surfaceforming Nickel Oxide [57].
MCFCs are typically used in stationary energy generation and industrial power generating [58]. The best commercially available MCFC can produce power ranging from 300 kW to 2.8 MW [56]. In the past, integrations of MCFC with solid oxide fuel cells (“SOFC”) for industrial power generation were proposed and studied [59]. Due to its extreme temperature requirements, MCFC applications such as home power generation and vehicle engines are considered implausible.
2.4. Solid oxide fuel cell
The fuel utilized to generate energy in solid oxide fuel cells (“SOFC”) is hydrogen and carbon monoxide. A methane steam reforming operation can provide this fuel combination. In the anode, hydrogen and carbon monoxide react with oxygen ions to produce water, carbon dioxide, and electrons. The electron goes from the anode to the cathode via an outside circuit, which generates electricity. Electrons in the cathode react with oxygen to form oxygen ions, which move back to the anode via an electrolyte [60]. The mechanism diagram of SOFC can be seen on Fig. 2d. The fundamental advantage of solid oxide fuel cells is that they can function at extremely high temperatures. The main disadvantage of using this type of fuel cell is the fact that carbon dioxide is still produced, so it is not exactly a form of clean energy. The main difference between SOFC and PEMFC is the electrolyte, where a proton exchange membrane is used for PEMFC while a solid oxide electrolyte is used for SOFC. Operating temperatures of SOFC are also extremely high in comparison to PEMFC which is made possible by the solid oxide electrolyte. Comparison of the various types of fuel cells could be seen in Table 1.
Table 1. Comparison of various types of fuel cells.
| FC Types | Catalyst | Membrane/Electrolyte | Fuel | Temperature | Advantages | Disadvantages | Power Range | Ref. |
|---|---|---|---|---|---|---|---|---|
| Proton exchange membrane fuel cell (PEMFC) | Pt | Nafion | H2 | ∼80 °C |
|
|
1 W-500 kW | [61] |
| Direct alcohol fuel cell (DAFC) | Pt/Ru (1:1) | Nafion | Methanol, ethanol | >60 °C |
|
|
100 mW-1 kW | [62] |
| Molten carbonate fuel cell (MCFC) | Ni or Ni Alloy | Molten carbonate | H2/CO/CH | 550–700 °C |
|
|
<1 kW-1MW | [63] |
| Solid oxide fuel cell (SOFC) | Ni or Ni Alloy |
Yttria Stabilized Zirconia (YSZ) |
H2/CO/CH | 700–1000 °C | 5 kW-3 MW | [64] |
3. Polyionic liquids
Ionic liquids (ILs) are salts that have a melting point below the temperature of 100 °C. ILs have been a hot topic of discussion and research because of their properties, such as their high electrical conductivity, thermal stability, and resistance to poisoning, such as by carbon monoxide [[65], [66], [67], [68], [69], [70], [71], [72], [73]] [[65], [66], [67], [68], [69], [70], [71], [72], [73]] [[65], [66], [67], [68], [69], [70], [71], [72], [73]]. These properties of ILs make them a strong candidate for applications in electrochemical energy devices [74]. Using ILs for this type of application has the potential for more durable, safer, and better performing energy generation.
Because most ILs are made up of organic ions, they are straightforward to adapt to suit their intended purpose and have a large range of potential configurations [75]. However, changes to the structure of an IL will change its physiochemical properties. Polymerization of ILs results in a structure known as PILs, which is an example of IL modification. PILs are reported to be more heat resistant and to have more mechanical strength than IL monomers. IL polymerization also overcomes several practical concerns with ILs, such as leaking or crossover of operation fluid or fuel.
PILs are polyelectrolytes which are made up of sets of IL monomers which are interconnected by polymerization, which forms a macromolecular structure. PIL could be formed by the polymerization of IL monomers. The physiological properties of PILs and their IL monomers are generally similar, but they are not always correlated [76]. Although the ionic structure of an IL can be easily modified into various structures, this could result in an alteration of their physiochemical properties [77]. Sometimes polymerization of IL into PIL sacrifices some advantageous property of the IL while improving other advantageous properties of the IL. PIL combines some elements of an ionic polymer with properties of an IL such as flammability, high ionic conductivity, adaptive solubility, and low temperature of glass transition [78]. The ease of access to synthesis materials and processes of IL and IL's flexible properties and structures make them a great candidate for membrane materials in the proton exchange membrane fuel cell.
The main synthesis method for producing PIL membranes is template synthesis. The templating method is commonly used not only for PIL membranes but also for polymer membranes in general, especially perforated membranes. PILMs are generally synthesized by in situ polymerization of IL monomers or copolymerization of ILs with other materials using the template method [[79], [80], [81], [82], [83]] [[79], [80], [81], [82], [83]] [[79], [80], [81], [82], [83]]. The template synthesis membrane has 2 main routes, which are synthesis by using a hard template and a soft template.
The hard template method gives a lot of control over the particular size and shape of the membrane's pores. When using colloid crystal as a hard template, a structure that appears like uniform colloid particles can be obtained [78]. One of the methods that implements the hard template is as has been done by Wilke et al., who succeeded in synthesizing a mesoporous membrane using a hard template from porous silica colloid crystals which that filled with IL additives and continued with cross-linking and etching. The membranes synthesized appear to have an increase in surface area (150–220 m2 g−1), clearly defined and uniform distribution of pores, small pore size distribution, and an increase in CO2 adsorption [[45], [84], [85]] [[,][84]] [[,][85]]. This method requires a high degree of cross-linking for a more stable mesoporous polymer structure towards damage caused after the removal of the silica template [79]. Steps needed to be taken before the synthesis process are silica crystal colloid fabrication template, IL monomer mixture template infiltration, cross-linker and initiator, followed by selective polymerization and dissolution of the silica template.
In general, an initiator is used to polymerize IL to generate PIL. AIBN is an example of an initiator. AIBN creates a chain that can join the IL monomers laterally, adding dimension to the PIL and potentially resulting in a stronger chain. The mechanism of IL polymerization may be shown in Fig. 4a, which depicts a membrane consisting of PIL [ViBuIm]TFSI and polymer 6FPBI. The IL not only connects the 6FPBI monomers together, but it also produces its own polymer and interacts with the FPBI-IL chain laterally via AIBN [86].

 Fig. 4. (a) Polymerization mechanism of 6FPBI - [ViBuIm]TFSI (adapted from Ref. [86]); (b): copolymerization reaction of the IL [HSO3-BVIm][TfO] and MMA (adapted from Ref. [87]); (c): Synthesis of IL Monomer 1-(4-Vinyl-benzyl)2,3-butyl imidazolium tetrafluoroborate (VBBIT) (adapted from Ref. [88]); (d): Synthesis of the PAMPS and PSS phosphonium based PIL; and (e) Synthesis of the PSTFSI phosphonium based PIL (adapted from Ref. [89]).
Fig. 4. (a) Polymerization mechanism of 6FPBI - [ViBuIm]TFSI (adapted from Ref. [86]); (b): copolymerization reaction of the IL [HSO3-BVIm][TfO] and MMA (adapted from Ref. [87]); (c): Synthesis of IL Monomer 1-(4-Vinyl-benzyl)2,3-butyl imidazolium tetrafluoroborate (VBBIT) (adapted from Ref. [88]); (d): Synthesis of the PAMPS and PSS phosphonium based PIL; and (e) Synthesis of the PSTFSI phosphonium based PIL (adapted from Ref. [89]).High proton conductivity and thermal stability are two qualities of membranes employed in PEMFC applications that are thought to be useful. The nafion membrane is a popular membrane used in PEMFC. Nafion's proton conductivity is largely dependent on the presence of water, which means that the membrane's performance degrades at higher temperatures due to the vaporization of water, which causes dehydration of the membrane. This problem is predicted to be solved by including PIL membranes into the membrane synthesis used in PEMFC applications.
A PIL membrane is typically created by combining a PIL with a polymer backbone. Materials usually used as polymer backbones include Nafion, polybenzimidazole, and sulfonated polyether ketone. These polymers need to have sufficient mechanical and chemical stability along with a great proton conducting capability. Some membranes also need additional components such as phosphoric acid, which are integrated by submerging the membrane for a certain amount of time in phosphoric acid. This is sometimes done to increase proton conductivity in some membranes with strong mechanical stability but relatively low conductivity, such as PBI. Characterization of the synthesized membrane is then done to study the membranes’ properties. These tests include conductivity test, mechanical stability test, FTIR or NMR tests, and fuel cell performance test.
4. PIL membranes synthesis and characterization
4.1. Membrane synthesis
There are several methods PIL could be introduced into membranes for proton exchange membrane fuel cells. The most frequent method is to mix a PIL with a regular polymer membrane [32]. The mix can be adjusted by changing the weight percentage of the PIL in comparison to the membrane to determine how much PIL make-up is best for PEMFC purposes. This chapter explains the process used to produce PIL membranes.
An example of PIL application in synthesizing a proton exchange membrane was in research done by Liu et al. 0.1 mol of 1-vinylimidazole and 0.12 mol of chlorobutane were reacted with and heated while being stirred at 60 °C for 72 h in a nitrogen atmosphere. The unreacted reactants were removed with toluene and acetone to extract [ViBuIm]Cl from the solution. [ViBuIm]Cl is then dried for 24 h [ViBuIm]Cl is then reacted with LiTFSI to undergo an anion exchange reaction by dissolving the solutions in deionized water. The product [ViBuIm]TFSI is then dried at 50 °C for 24 h 0.09 mol of [ViBuIm]TFSI is then reacted with 0.01 mol of allyl glycydyl ether and 0.3%-wt of AIBN. The mixture is heated in an oil bath at 70 °C for 24 h to complete the polymerization process. The PIL membrane is then dried at 50 °C for 24 h. In the research, the PIL is reacted by solvent casting using DMAc and the polymer 6FPBI at 90 °C for 24 h and then crosslinked at 150 °C in a vacuum oven for 9 h [86]. The membrane was then dried to remove any remaining solvent. The membrane was then immersed in KOH solution before being doped with phosphoric acid [35].
A membrane prepared from 1-(4-sulfobutyl)-3-vinylimidazoliumtrifluoromethane sulfonate or [HSO3-BVIm][OTf] has been done by Diaz et al. Firstly, the IL was prepared by dissolving 1-vinylimidazole in acetonitrile inside a round flask in an inert atmosphere. The solution was then cooled using ice at a freezing temperature and a solution of 1,4-butane sulfone was added. The solution was stirred and constantly heated at 85 °C for 5 days. Correspondingly, a precipitate of zwitterion was obtained by filtration of the solution. The obtained zwitterion is washed using diethyl ether before being dried. At room temperature, the high purity zwitterion was dissolved in acetonitrile. Trifluoromethanesulfonic acid was then added to the solution, which was then stirred for 5 h. The solvent was then removed and the intended IL was obtained and dried [90]. Preparation of the film using the IL was done by a method previously used by Bara et al. The membrane was put in a petri dishbefore the crosslinking agents divinylbenzene and 2-hydroxy-2-methylpropiophenone were added. The mixture was then dissolved in ethanol. The solvent was then removed by evaporation for 4 h, and the membrane was obtained [91].
A membrane composite made of fluorine-containing-polybenzimidazole and a crosslinked PIL membrane was synthesized by Liu et al. First, 20 mmol of 3,3′-diaminobenzidin was dissolved in 173.28 g of polyphosphoric acid to make 6FPBI. The solution was stirred until a homogenous solution was obtained. 2,2-bis(4-carboxyphenyl) hexafluoropropane and diphosphore pentaoxide is added to the solution, then the solution was heated at 200 °C for 8 h. The mixture was then poured into deionized water from which a polymer was obtained. The polymer is then dried at 100 °C for 24 h. The next step is to prepare the crosslinked PIL which is the [ViBuIm]TFSI. [ViBuIm]Cl was first obtained by mixing 0.12 mol of 1-vinylimidazole and 0.12 mol of 1-chlorobutane at 60 °C for 72 h. An anion exchange reaction was carried out using an IL and the lithium salt of bis-(trifluoromethanesulfonyl) imide in deionized water. The obtained IL was then copolymerized with AIBN and then dried at 50 °C for 24 h. The 6FPBI polymer was added to DMAc along with the polymer of AIBN and the PIL. The solution casting method was then carried out on a glass plate at 90 °C for 24 h in a vacuum oven. The obtained membrane was then heated at 150 °C for 9 h for the crosslinking process [86,92,93].
Ortiz-Martinez et al. synthesized copolymer membranes from the protic IL [HSO3-BVIm][TfO] and ethyl methacrylate or MMA, as well as hydrolyzed fluoro-3,6-dioxa-4-methyl-7-octene sulfonyl fluoride, or hPFSVE. The PIL was created by first reacting 1-vinylimidazole with 1,4-butane sulfone, then adding an acetonitrile solution of triflic acid. By adding a basic solution such as KOH or NaOH and then filtering the solution to remove salts and evaporating the remaining solvent, fluoro-3,6-dioxa-4-methyl-7-octene sulfonyl fluoride, or PFSVE monomer, was hydrolyzed to convert SO2F groups to sulfonic acidgroups. The hydrolyzed monomer was polymerized in an autoclave at −90 °C and 20 bar pressure using the initiator Vinylidene Fluoride (VDF) [94]. The copolymerization of the polymer membrane and the IL was done by in-situphotopolymerization by adding a quantified ratio of the PIL, either the MMA or hPFSVE, cross-linker glycerol dimethacrylate and photoinitiator 2-hydroxy-2-methyl propiophenone [87]. Copolymerization of the IL with MMA could be seen in Fig. 4b.
Hu et al. prepared a novel polymer electrolyte by grafting PIL 1-(4-Vinylbenzyl) 23-butylimidazolium tetrafluoroborate (VBBIT) onto silica nanoparticles. The PIL was prepared by first dissolving 50 mmol of 1-Butylimidazole in 40 ml of CHCl3. This is followed by the addition of 55 mmol of vinylbenzyl chloride and 0.25 mmol of the polymerization inhibitor, 2,6-di-tert-butyl-4-methylphenol. The solution was stirred at 50 °C for 8 h. The solvent was then removed using a rotary evaporation and the obtained liquid was washed using 25 ml of diethyl ether and then dried overnight at vacuum pressure. The yield obtained is 85% VBBICl. The VBBICl is then dissolved in 60 ml of dry acetone alongside 0.2 mmol of polymerization inhibitor. 48 mmol of sodium tetrafluoroborate is added slowly and the solution is stirred for 3 days. The solution was filtered and the acetone was removed by evaporation, while the VBBICl was separated by washing with distilled water and diethyl ether. An ATRP initiator modified silica nanoparticle was obtained by dissolving 2.2 mmol of (11-(2-Bromo-2-methyl) propionyloxy) undecyltrichlorosilane and silica nanoparticles in toluene. VBBITis obtained by anion exchange of VBBICl and NaBF4. VBBIT and surface initiator PMDETA were dissolved in butyronitrile. CuBr is then added under a nitrogen atmosphere and the reaction is conditioned to be at 90 °C and under magnetic stirring for 16 h. The PIL product is washed with acetone and DMF and dried under vacuum pressure at 60 °C. The silica nanocomposites and the PIL were mixed at a 4:1 ratio and stirred for 24 h. The obtained solution was cast onto a glass substrate and heated at 60 °C for half an hour before cooling down [88]. Synthesis of the IL monomer VBBIT could be seen in Fig. 4c.
Rewar et al., prepared a proton exchange membrane by blending polybenzimidazole and PIL into poly-diallyldimethylammonium TFMS or P[DADMA][TFMS]. Polybenzimidazole could be prepared by polycondensationof 3,30-diaminobenzidine and isophtalic acid or obtained commercially. The PIL P[DADMA][TFMS] is prepared by anion exchange of the commercially available P[DADMA][Cl]. The polybenzimidazole was dissolved in DMAc at 80 °C while the PIL was dissolved in DMSO with continuous stirring for 12 h. The two solutions were mixed and stirred for 24 h. The solution was cast on the glass surface and heated to 90 °C for 24 h. The film was dried in a vacuum oven at 80 °C for 8 days to remove residual solvent [95].
Li et al. prepared an anion exchange membrane by constructing ion nanochannels by in-situ assembly of PILs within a metal organic framework. The first step is the construction of the MIL-101 matrix by a method called the hydrothermal method. 4 g of chromium nitrate and 1,67 g of terephtalic acid are dissolved in 48 mililitres of water. The solution is kept in a hydrothermal synthesis reactor at 220 °C for 8 h. The turbid liquid obtained is centrifuged to get the MIL-101. The crude MIL-101 is then washed and submerged in N,N-dimethyl formamide or DMF to remove the unreacted terephtalic acid and Chromium Nitrate. The obtained crude MIL is treated by using ethanol and dichloromethane under reflux to replace the high boiling point solvent. N-vinyimidazolium and vinylbenzylchloride are precursors of the PIL poly N-vinylimidazolium. The PIL was inserted into the MIL by a method called the “ship in a bottle” method. The MIL was first degassed in a schlenk tube at 120 °C at vacuum pressure for 2 days. PIL precursors were added to the schlenk tube after degassification. The schlenk tube's pressure was then increased to allow the MIL to suck the PIL precursors. The mixture is stirred for 2 days to allow an even distribution. The mixture was washed to remove excess precursor and separated into a paste by centrifugation. The paste was treated at 60 °C and a powder of PIL@MIL was obtained. The PIL@MIL is bound with a 95:5 PIL:SEBS ratio of hydrogenated styrene-ethylene/butylene-styrene triblock copolymer or SEBS. The mixture was continuously ground and the powder was tiled and pressed at 100 °C under 20 MPa pressure. After cooling down and being demolded, the PIL@MIL membrane is obtained [96,97].
Koyilapu et al. prepared a nanocomposite based proton exchange membrane by grafting vinylimidazolium PIL onto silica nanoparticles through the reversible addition-fragmentation chain transfer or RAFT polymerization method. Polymerization of the IL monomer VImBr was done in a schlenk tube on the surface of a composite made of synthesis of silica nanoparticles or siNP and synthesis of RAFT agent CPDB. A reaction between SiNP-CPDB, the IL monomer, free CPDB and AIBN is done inside a schlenk tube where 3 freeze-pump-thaw cycles followed by sonication for 10 min are applied and the reaction is stirred for 16 h in a 70 °C oil bath. The mixture obtained from the reaction is quenched with nitrogen and precipitated with acetone and diethyl ether. The product was redispersed in methanol and then re-precipitated and centrifuged at 7000 rpm for 30 min. The step was repeated until a PVImBr grafted SiNP PVImBr-g-SiNP was obtained which was then dried at 70 °C under vacuum pressure for 48 h. The membrane PVImBr-g-SiNP is then dispersed in methanol along with aqueous HF and the mixture is stirred for 12 h. The polymer is then extracted with methanol and excess HF is neutralized with NaHCO3. The nanoparticles are then added to a polymer solution in formic acid and stirred for 24 h. The membrane thus obtained is then dried in an oven and doped with phosphoric acid [98].
Isik et al. created a proton conducting membrane from protic PILs with phosphonium counter-cations. Poly(styrene sulfonic acid) (PSS), poly(2-acrylamido-2-methyl-1-propane sulfonic acid) (PAMPS), and poly[(4-styrene-nesulfonyl) (trifluoromethanesulfonyl)imide] (PSTFSI) were the polymeric backbones utilized to make the PILs. Ributylphosphine (P444), tri-octylphosphine (P888), and tricyclohexylphosphine (PCyCl3) were utilized in combination with the polymer backbone. The protic polymers were dissolved in a methanol/water mixture and neutralized for 14 h with phosphines for the PSS and PAMPS. The solvent was evaporated to obtain the protic PIL. Protic PIL is generated for the PSTFSI through cation exchange of lithium cation and protic-phosphonium, which is driven by precipitation of the hydrophobic PIL [89]. Synthesis of PAMPS, PSS, and PSTFSI phosphonium based PIL could be seen in Fig. 4d and e.
Qiu et al., prepared a membrane based on Bis-imidazolium IL that was photocrosslinked into an anion exchange membrane. Firstly, 1-Butyl-2-methylimidazole was synthesized by dissolving 2-methylimidazole, 1-bromobutane and NaOH in acetonitrile and stirring it at room temperature for 4 h. The mixture is then filtered and the resulting filtrate is dissolved in deionized water and then extracted using methylene dichloride. The mixture is then evaporated at 30 °C and the product is obtained. 1-allyl-2-methylimidazole is obtained using the same method but using different materials, which are 2-methylimidazole, allyl bromide, and NaOH. 1-Butyl-2-methylimidazole is then dissolved in a 25:75 M ratio with 1, 6-dibromohexane in ethanol at 50 °C for two days. The solvent is then evaporated and the resulting viscous liquid is washed with ethyl acetate and ethylether. The residue was purified using silica gel column chromatography with methanol/acetone. 3-(6-bromohexyl)-1-butyl-2-methylimidazol-3-ium bromide, also known as [BHBMIMI][Br], is synthesized. 1-allyl-2-methylimidazole and [BHBMIMI][Br] are dissolved in ethanol in a 60:40 M ratio at 50 °C for two days. The mixture is then treated the same as the viscous oil obtained during the synthesis of [BHBMIMI][Br]. 1-allyl-3-(6-(1-butyl-2-methylimidazol-3-ium-3-yl)hexyl)-2-methylimidazol-3-ium bromide or [ABMHM][Br]2 is obtained. The anion exchange membrane is prepared by mixing [ABMHM][Br]2, divinylbenzene, 2%-wt benzoin ethylether, and styrene/acrylonitrile mixture and then stirring and ultrasonicating the mixture to obtain a homogenous solution. The solution was then cast onto glass and photocrosslinked using ultraviolet light at room temperature. The membrane is then bathed in nitrogen saturated KOH solution at 60 °C for 24 h, converting the Br− to OH− resulting in [PABMHM][OH]2. The process is done in three iterations and the membrane is obtained [[99], [100], [101]] [[99], [100], [101]] [[99], [100], [101]].
Vilela et al., prepared an anion exchange membrane based on PILs and bio-based nanocellulose. The wet bacterial nanocellulose's water content was first removed using absorbent paper. The BC was then stoppered and then purged with nitrogen in an Erlenmeyer. Aqueous solutions of the IL monomer methacroylcholine chloride, the cross-linker MBA, and the radical initiator AAPH with 1%-wt of the monomer were prepared. The solutions were then added to BC in the initial Erlenmeyer according to the planned composition. The mixture was then left for 1 h on ice for complete incorporation. The solution was then heated up at 70 °C for 6 h before being washed and dried in an oven. The membranes then go through ion exchange with an aqueous basic solution of 1 M NaOH for 24 h. The membranes were then dried again and kept in a desiccator. Five membranes were prepared. Membrane A has a 2:1 ratio of MACC and BC with no crosslinker. Membrane B has a 2:1 ratio of MACC and BC with a 0.05:1 ratio of crosslinker to the IL monomer. Membrane C has a 5:1 ratio of MACC and BC with a 0.05:1 ratio of crosslinker to the IL monomer. Membrane D has an 8:1 ratio of MACC and BC with a 0.05:1 ratio of crosslinker to the IL monomer. Membrane E has a 10:1 ratio of MACC and BC with a 0.05:1 ratio of crosslinker to the IL monomer [102].
4.2. Membrane characterization
Liu et al. studied how the addition of the polymerized IL [ViBuIm]Cl to fluorine-containing polybenzimidazole would affect the membranes' phosphoric acid retention. The morphological assessment of the membrane through FTIR shows the result of the composition of the IL and the polymer. The N–H stretching bond of the imidazole ring at 3300–3500 revealed the presence of the PIL [103]. The composite membranes could be confirmed by missing peaks at 2856, 2994, and 3066, and 1645, 998, and 918, which show missing saturated and unsaturated C–H stretching and C C and epoxy groups [[86], [104], [105]] [[,][104]] [[,][105]]. The pristine FPBI membrane experiences thermal degradationin two steps, which are at 300–400 °C which is solvent removal, and at 500 °C which is degradation of the polybenzimidazole polymer backbone. The addition of PIL to the membrane matrix introduces a new degradation step at 250–450 °C which is attributed to the degradation of PIL [[95], [106]] [[,][106]]. Doping of the FPBI membrane and the addition of PIL significantly weakens the membrane's tensile strength and makes the membrane more elastic. This is shown by a decrease in the maximum stress that is applicable to the membrane and an increase in the membrane's strain. The addition of phosphoric acid causes the hydrogen bonds in the polymer matrix to break, which in turn causes a decrease in the mechanical strength of the membrane [107].
C and epoxy groups [[86], [104], [105]] [[,][104]] [[,][105]]. The pristine FPBI membrane experiences thermal degradationin two steps, which are at 300–400 °C which is solvent removal, and at 500 °C which is degradation of the polybenzimidazole polymer backbone. The addition of PIL to the membrane matrix introduces a new degradation step at 250–450 °C which is attributed to the degradation of PIL [[95], [106]] [[,][106]]. Doping of the FPBI membrane and the addition of PIL significantly weakens the membrane's tensile strength and makes the membrane more elastic. This is shown by a decrease in the maximum stress that is applicable to the membrane and an increase in the membrane's strain. The addition of phosphoric acid causes the hydrogen bonds in the polymer matrix to break, which in turn causes a decrease in the mechanical strength of the membrane [107].
Liu et al. also studied how the IL [ViBuIm]TFSI could help the phosphoric acid uptake and retention and the 6FPBI membranes’ proton conductivity. The [ViBuIm]TFSI structure was confirmed by H NMR chemical shifts at 7.93 ppm, 8.18 ppm, and 9.50 ppm, which were attributed to the attachment of imidazolium cations to the 6FPBI membranes. FTIR analysis shows that N–H stretching of the 6FPBI imidazole ring is evidenced by the peak at 2800–3400. The imidazolium ring of the crosslinked composite membrane is at the absorption peak of 3152. Missing peaks at 918 and 953 for the composite membranes show the opening of the epoxy group. Observation of the cross-section of the 6FPBI and 6FPBI-cPIL-10 membranes showed that incorporation of PIL enhances the toughness of the membrane and makes the sample have a rough fracture cross-section. More compact chemical structure of the membranes also enhances the mechanical strength.
As previously mentioned, the pristine membrane shows a 2-step degradation while the composite membrane shows a 4-step degradation. In addition to solvent removal and polymer degradation, 2 new steps at temperatures of 350 and 530 °C are the decomposition of the IL's anion TFSI and the crosslinked PIL's backbone, respectively [95,106]. For the doped membrane solvent removal, which in this case is the phosphoric acid, appears at 160 °C because of PA dehydration and also the formation of polyphosphoric acid [108]. Mechanical testing of the membranes shows that an increase in cPIL content leads to a lower tensile strength and higher elongation before break, which suggests an elastic property. The membranes' ability to uptake phosphoric acid also affects their mechanical strength, which is the case for 6FPBI-cPIL-10, which has the highest PA uptake and also the lowest mechanical strength. The membrane considered most optimum is the 6FPBI-cPIL-20 membrane with tensile strengths of 119 MPa and 2.98 MPa when doped and undoped, respectively [86]. SEM morphology and TG analysis of the membrane could be seen in Fig. 5a–c.
 Fig. 5. (a) SEM of cross-section morphology of 6FPBI, (b) 6FPBI-cPIL-10, and (c) thermogravimetric analysis of 6FPBI - [ViBuIm]TFSI, reprinted with permission from Ref. [86]. (d): Thermogravimetric analysis and FTIR Spectra of poly([HSO3-BVIm][TfO]/[HSO3-BMIm][TfO] membranes, reprinted with permission from Ref. [109].
Fig. 5. (a) SEM of cross-section morphology of 6FPBI, (b) 6FPBI-cPIL-10, and (c) thermogravimetric analysis of 6FPBI - [ViBuIm]TFSI, reprinted with permission from Ref. [86]. (d): Thermogravimetric analysis and FTIR Spectra of poly([HSO3-BVIm][TfO]/[HSO3-BMIm][TfO] membranes, reprinted with permission from Ref. [109].