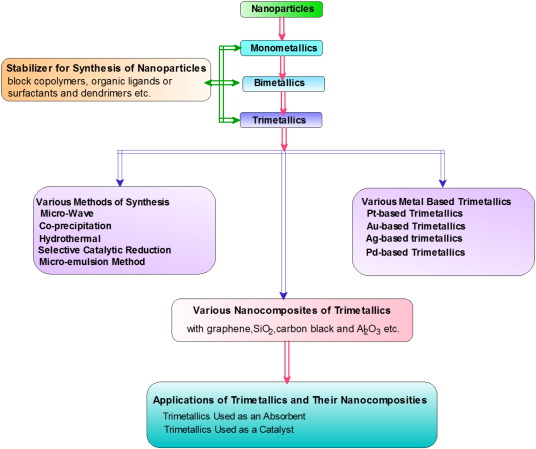
1.1. General introduction on nanoparticles
The nanoparticles (NPs) may be defined as a distinctive nano-object where all the three Cartesian dimensions are less than 100 nm. These are larger than atoms or molecules individually but smaller than bulk solids. The physical and chemical properties of nano-scaled particles and bulk material are different [1], [2]. Another name used for NPs is nanoclusters and “soft” bio-organic nanoparticles. A nanocluster is combination of the same sized particles which are of nanometer dimensions. The nanoparticles (NPs) and nano metal clusters exhibit an important state of condensed matter. However nanoparticles show a gap between the atomic or molecular structure and bulk materials. They show a number of special properties such as high surface area to volume ratio which gives outstanding force for diffusion etc. The nanoparticles find new applications in different fields such as non-linear optics, battery cathodes and ionics, sensors, nano-wires and other systems. As electronic display system has been improved by the metal nanoparticles, which takes the most important position in information technology. The metallic nanoparticles are of different sizes and these are synthesized by chemical approach and their surfaces are stabilized by special functional groups. They are found to be very significant in many fields such as semiconductors [3], electro-optic materials [4], magnetic materials [5], [6], [7], catalysis [8], [9], [10], [11] and drug delivery system [12], [13], removal of organic pollutants [14], [15], [16], antimicrobial activity [17], [18], [19] and removal of toxic metal ions etc. [20], [21], [22]. Scheme-1represents the revolution from monometallic to trimetallic nanoparticle composites, their various synthesis methods and applications.
 Scheme 1. Representation of revolution from monometallic to trimetallic nanoparticle composites, various synthesis methods and applications.
Scheme 1. Representation of revolution from monometallic to trimetallic nanoparticle composites, various synthesis methods and applications.1.2. Monometallics to trimetallics
From the monometallics, the physical mixture of nanoparticles likes Ru Pt in solution shows more catalytic movement than the equivalent monometallic nanoparticles. From single or simple monoatomic catalysts the designable history of catalytic material show an improvement specially Pd, Pt and Au which is in nano dimensioned to bimetallic structure. It has been seen that physical mixture of Ag, Pd, Rh, and Pt, rapidly forms the bimetallic nanoparticles with Au-core structure in aqueous solution. The main reason for using Ag and Rh nanoparticles is the prominent individuality of Rh nanoparticles as a catalyst. On the other hand, the probable electronic effect of silver is similar to gold upon improvement of the catalytic movement with Rhodium. In addition, Ag is cheap metal compared with Au. The colloidal dispersions of Ag and Rh metallic nanoparticles were protected by (PVP) poly (N-vinyl-2-pyrrolidone). The polymers which are water soluble were synthesized by an alcohol reduction method. The average diameters of Rh and Ag monometallic nanoparticles were 2.2 nm and 7.5 nm. The catalytic movement of external metal has been enhanced by the bi-metallization of metal nanoparticles. Several study established that the Au-core/Pt shell metal nanoparticles demonstrate the superior catalytic movement for the visible-light induced hydrogen generation than Pt monometallic nanoparticles. The formation of the Ag-core bimetallic nanoparticles is extensively reviewed in present times. The Au/Pt bimetallic system has been stabilized by using polymer. The study has been performed for the hydrogen generation using Au/Pt nanoparticle catalyst. On the other hand, degradation of poly chlorinated biphenyls (PCBs) in aqueous solution was examined with the support of bimetallic catalyst Fe-C/Pd gives of palladized grey cast iron. And with a recently designed trimetallic catalyst (Cu-Fe-C/Pd) giving of a copper metal plate with embodied microparticles of palladized grey iron (bimetallic catalyst). PCB solutions were synthesized by using DELOR 103. The trimetallic catalysts were considerably more professional than bimetallic ones due to their unique structural composition and arrangements. Trimetallic catalyst reduces the concentration of PCBs to just about 1% of its original value during 70 min of contact and bimetallic one in 48 h. Additionally, more than 20% of declined PCBs were found adsorbed on the surfaces of the bimetallic catalysts. The presence of copper metal accelerates the enhancement of oxidation product. It discharges from the catalyst surface as individual flock, which recovers the movement of trimetallic catalyst.
Pt in solution shows more catalytic movement than the equivalent monometallic nanoparticles. From single or simple monoatomic catalysts the designable history of catalytic material show an improvement specially Pd, Pt and Au which is in nano dimensioned to bimetallic structure. It has been seen that physical mixture of Ag, Pd, Rh, and Pt, rapidly forms the bimetallic nanoparticles with Au-core structure in aqueous solution. The main reason for using Ag and Rh nanoparticles is the prominent individuality of Rh nanoparticles as a catalyst. On the other hand, the probable electronic effect of silver is similar to gold upon improvement of the catalytic movement with Rhodium. In addition, Ag is cheap metal compared with Au. The colloidal dispersions of Ag and Rh metallic nanoparticles were protected by (PVP) poly (N-vinyl-2-pyrrolidone). The polymers which are water soluble were synthesized by an alcohol reduction method. The average diameters of Rh and Ag monometallic nanoparticles were 2.2 nm and 7.5 nm. The catalytic movement of external metal has been enhanced by the bi-metallization of metal nanoparticles. Several study established that the Au-core/Pt shell metal nanoparticles demonstrate the superior catalytic movement for the visible-light induced hydrogen generation than Pt monometallic nanoparticles. The formation of the Ag-core bimetallic nanoparticles is extensively reviewed in present times. The Au/Pt bimetallic system has been stabilized by using polymer. The study has been performed for the hydrogen generation using Au/Pt nanoparticle catalyst. On the other hand, degradation of poly chlorinated biphenyls (PCBs) in aqueous solution was examined with the support of bimetallic catalyst Fe-C/Pd gives of palladized grey cast iron. And with a recently designed trimetallic catalyst (Cu-Fe-C/Pd) giving of a copper metal plate with embodied microparticles of palladized grey iron (bimetallic catalyst). PCB solutions were synthesized by using DELOR 103. The trimetallic catalysts were considerably more professional than bimetallic ones due to their unique structural composition and arrangements. Trimetallic catalyst reduces the concentration of PCBs to just about 1% of its original value during 70 min of contact and bimetallic one in 48 h. Additionally, more than 20% of declined PCBs were found adsorbed on the surfaces of the bimetallic catalysts. The presence of copper metal accelerates the enhancement of oxidation product. It discharges from the catalyst surface as individual flock, which recovers the movement of trimetallic catalyst.
1.3. Triple core-shell structure
From the concept of electronic effect in trimetallic nanoparticles a “triple core-shell structure” in which one element forms a core and second element covers the core and its forms an interlayer. The third element covers the interlayer which is formed by the second element [2]. The second metal cannot help tailor sought after properties in terms of surface morphology, shape and size of particles. But it can accelerate the catalytic effectiveness by the movement of electron density from the original hold metal into surface catalytic organization. The colloidal metallic nanoparticles are rising as major families of multifunctional nanoscale materials that found a variety of applications [23], [24], [25], [26], [27], [28]. Because, in metal catalysts, addition of other element can frequently get better the catalytic selectivity and activity. From the same point of view trimetallic and bimetallics nanoparticles (NPs) are also investigated, the fact that their nanoarchitectures, compositions, and particle sizes can be simply adjusted by convenient method to regulate their properties in fundamental manner [29], [30], [31], [32]. Triple core-shell particles have been developed with significant feature to give important perspectives, for effectively changing the functionalities of bimetallics and trimetallic nanoparticles. Colloidal metal hybrid nanoparticles [33], [34], [35], [36] are proficient as an important family of multifunctional nanoscale apparatus that support various applications in sensors [37], [38], [39], [40], optics [41], solar energy conversion [42], [43], cancer therapy [44], biological imaging [45], [46], catalytic properties [47], [48], [49], [50] and magnetic properties etc. [51].
1.4. Synthesis of monometallic and bimetallic nanoparticles
Bimetallic nanoparticles are of huge attention than that of monometallic due to their unique architectures designed for specific applications. As reported that bimetallic nanoparticle has better stability and catalytic properties than that of the single metal catalyst. Both bimetallic and monometallic nanoparticle can be synthesized by different approaches. Metal nanoparticle can be synthesized by two ways by part of bulk metals (a physical method) and enlargement of particle from molecular precursors (chemical method). The chemical method is more appropriate than that of the physical methods as the size and stability of the metal nanoparticles can be tailored by the chemical method.
1.5. Synthesis of trimetallics nanoparticles
The trimetallics nanoparticles have been synthesized by different methods including the microwave, co-precipitation, hydrothermal, selective catalytic reduction and micro emulsion etc. The microwave (MW) dielectric heating is very advantageous method for synthesis as by using it the structural aspect can be tailored by controlling the reaction conditions. For example Au-Pt-Ag trimetallic nanocomposites have been synthesized by the microwave method. The co-precipitation is one of the simplest method employed for synthesis of various types of nanoparticles in diverse forms such as oxides, hydroxides, carbonates and sulphides etc. [52], [53], [54]. These precipitates are calcined at suitable temperatures to form the final powder. Co-precipitation is a most useful method in chemical analysis, where it is frequently unnecessary, but it can be exploited in few cases. However, co-precipitation is complicated phenomena because undesired impurities frequently co-precipitate with the analyte, subsequently as additional mass. For example trimetallic nanocomposite CeO2ZnO-ZnAl2O4 has been synthesized by co-precipitation method. On the other hand, hydrothermal technique can be defined as a method of synthesis of single crystals which depends on the solubility of minerals in hot water under high pressure. The crystal growth is performed in an apparatus having a steel pressure vessel called autoclave. In autoclave, the nutrient is supplied along with water. A gradient of temperature is maintained at the opposite ends of the growth chamber so that the hotter end dissolves the nutrient and the cooler end causes seeds to take additional growth. For example the trimetallic Pd1NiyAl0.5 nanoparticles have been synthesized by hydrothermal method. The selective catalytic reduction (SCR) is one of another important method used for synthesis of mono, bi and trimetallic nanoparticles. For example trimetallic (Aurod-Pdshell-Ptcluster) catalyst has been synthesized by selective catalytic reduction method. The micro-emulsion method can be defined as an isotropic system and thermodynamically stable system. It establishes the minor droplets distinct in an immiscible solvent and an amphiphilic surfactant species on the surface of the micelle. The micro-emulsion mediated synthesis has been mainly used for the dimension selective synthesis and self-gathering of nanoparticles due to the excellent control on the particle size, which can performed through modification of micelle [55], [56], [57], [58]. For example the Pd-Co-Au trimetallics have been synthesized by micro-emulsion method.
1.6. Stabilizer for the synthesis of nanoparticles
The surfaces area of trimetallic nanoparticles is comparatively unstable and thus gets simply precipitated away from their solution. Hence, resulted in their reduced catalytic activity. By using stabilizers like block copolymers, organic ligands or surfactants and dendrimers the metal nanoparticles can be stabilized. A polymer gives stabilization for metal nanoparticle sterically by the largeness or bulk of their framework. PPO (poly (2, 5-dimethylphenylene oxide) and PVP (poly vinyl pyrolidone) are mainly used for nanoparticle stabilization and catalysis as these two complete both ligand and steric condition. Many other polymers have been freshly used as effective support for nanoparticle catalysis like polyacrylonitry/polyurea, polyacrylic acid and multilayer polymer.
2. Various metals based trimetallic nanoparticles
2.1. Pt-based trimetallics
Platinum nanoparticles are now being used in the upcoming generation for automotive catalytic converters due to reason that they have high surface area. Hence the amount of platinum required is less for their fabrication. The most important electrodes are Pt-based materials, even when kinetics of ORR (Oxidation Reduction Reaction) is slow; this causes loss in energy conversion devices [59], [60], [61], [62]. In present time, electrode materials research shows a great interest in developing and searching the platinum based materials for ORR (Oxidation Reduction Reaction). To enhance the efficiency of Pt-based electrodes, the Pt based trimetallic and bimetallic nanoparticles catalysts are synthesized [63], [64]. Core-shell structured catalyst, contain only small amounts of platinum and palladium, used in catalytic applications [65]. Among all the routes used, in the present time, to prepare Pt catalysts for direct methanol fuel cells(DMFC) and proton exchange membrane fuel cells (PEMFC) are micro emulsion [66], [67], impregnation [68] and colloidal [69] etc. The urea burning preparation appears as a promising a novel method to obtained very well (nanosized), crystalline powders in a quick, simple and costless procedure. In Pt-based catalyst studies, the addition of other metals to the Pt catalyst may not only decrease the utilization of noble metal Pt, but also improve the catalytic ability [70], [71]. For example, Hu et al. [70] has concluded that bimetallic Pt Au nano-catalyst shows better catalytic capability to single metal catalyst of Au or Pt. Nanoscaled Au is established to demonstrate exceptional electro catalytic activity for CO oxidation [72], [73], [74]. In addition, at high potentials, bimetallic nanoparticles such as Pt and Ru catalyst are less stable than the Pt Pd catalyst. Consequently, the arrangement of three metals of Au, Pd and Pt is expected to give highly enhanced catalytic capability because of the synergistic catalytic effect of the three noble metals. Yu et al. [75] in 2013, had broken a shape recovery process in Pt
Au nano-catalyst shows better catalytic capability to single metal catalyst of Au or Pt. Nanoscaled Au is established to demonstrate exceptional electro catalytic activity for CO oxidation [72], [73], [74]. In addition, at high potentials, bimetallic nanoparticles such as Pt and Ru catalyst are less stable than the Pt Pd catalyst. Consequently, the arrangement of three metals of Au, Pd and Pt is expected to give highly enhanced catalytic capability because of the synergistic catalytic effect of the three noble metals. Yu et al. [75] in 2013, had broken a shape recovery process in Pt Ni bimetallic nanocrystal attributable to fault effects. Site selective nucleation of third metal around the fault to design trimetallics Pt3Ni@M core shell structure (M = Rh, Cu, Ag, Au) shown an intrinsic defect dominated growth mechanism. Tompos et al. [76] in 2007 synthesized the trimetallic Pt-Pd-Au/CeO2 catalysts for methane oxidation. Methane oxidation activity promoted by introduction of Pt/Pd, Au and it also improve the long term stability as compare to monometallic Pd catalysts. Fang et al. [77] 2011, designed the Pt cluster, Au core and Pd-shell for increasing the electro catalytic activity. On the other hand for oxidation of small organic molecules the Pt and Pd based catalyst are poisoned by intermediate products. The use of noble metal Pt can be reduced by synthesizing the Pt- based trimetallics material as they shows increased catalytic activity, like Pd1-Niy-Al0.5[78], Au-core Pd-shell Pt-cluster nanoparticles, Pt-Cu-Co [79] and Pt-Sn-Cu [80]etc. At high potential, Pt
Ni bimetallic nanocrystal attributable to fault effects. Site selective nucleation of third metal around the fault to design trimetallics Pt3Ni@M core shell structure (M = Rh, Cu, Ag, Au) shown an intrinsic defect dominated growth mechanism. Tompos et al. [76] in 2007 synthesized the trimetallic Pt-Pd-Au/CeO2 catalysts for methane oxidation. Methane oxidation activity promoted by introduction of Pt/Pd, Au and it also improve the long term stability as compare to monometallic Pd catalysts. Fang et al. [77] 2011, designed the Pt cluster, Au core and Pd-shell for increasing the electro catalytic activity. On the other hand for oxidation of small organic molecules the Pt and Pd based catalyst are poisoned by intermediate products. The use of noble metal Pt can be reduced by synthesizing the Pt- based trimetallics material as they shows increased catalytic activity, like Pd1-Niy-Al0.5[78], Au-core Pd-shell Pt-cluster nanoparticles, Pt-Cu-Co [79] and Pt-Sn-Cu [80]etc. At high potential, Pt Pd is more stable as compare to these materials. Greater movement of hydrogen oxidation reaction on platinum makes the platinum as a major catalyst for the proton exchange membrane fuel cells (PEMFCs) anode [81]. But, as it is known, that pure Pt at room temperatures is basically poisoned by CO, so upto date the efforts has been focused from the synthesis of a Pt alloy with better CO acceptance [82]. A few of the compositions at present study are Pt
Pd is more stable as compare to these materials. Greater movement of hydrogen oxidation reaction on platinum makes the platinum as a major catalyst for the proton exchange membrane fuel cells (PEMFCs) anode [81]. But, as it is known, that pure Pt at room temperatures is basically poisoned by CO, so upto date the efforts has been focused from the synthesis of a Pt alloy with better CO acceptance [82]. A few of the compositions at present study are Pt Mo [83], Pt
Mo [83], Pt Sn [84], [85], [86], Pt–Ru–Mo [87], [88] Pt–Ru–W, and Pt–Ru–Ni [89] etc. Pt–Ru–Co can be hypothetically regarded as a sufficient applicant for the substitution of Pt catalyst [90].
Sn [84], [85], [86], Pt–Ru–Mo [87], [88] Pt–Ru–W, and Pt–Ru–Ni [89] etc. Pt–Ru–Co can be hypothetically regarded as a sufficient applicant for the substitution of Pt catalyst [90].
2.2. Ni-based trimetallics
In non-noble metal, Ni is abundant in nature. The stability and activity of the catalyst has been increased due to synergistic effect between Ni and Pd Pt [91], [92]. Powders of copper, nickel and cobalt are broadly used for the reason that they possess good magnetic, catalytic and electronic properties. Investigations have been concluded that when one metal was associated with another metal in trimetallic form, the properties of resultant material can improved with respect to those of hygienic metals. Lately, there is a rising concern in the preparation of Ni-based alloy nanoparticles because of its exclusive selectivity [93] and catalytic movement [94] than monometallic nickel. Catalysts which have nickel are most commonly used because of low cost, fast turnover rate and higher stability [95]. Nickel metal particles have active sites [96]. This active sites increase the carbon deposition on surface of materials [97], [98]. Nickel catalysts are in general merit analysis because they are less luxurious than rhodium or palladium catalysts, a feature that is useful for significant experiments and for industrial applications. When we dilute the nickel metal with another metal like Cu, Fe, Al, Mn etc. we can also prevent the sintering of metal particles [99], [100], [101], [102], [103]. The nickel complexes are knowledgeable for their catalytic activity with cyclotrimerization of phenylacetylene. This reaction is more important in natural product synthesis. On the other hand when temperature is close to the reforming temperature Co or Ni formed the trimetallic, perovskites (La-Ni-Fe or La-Co-Fe) which can be reduced to form active metallic species [104], [105]. Trimetallic Ni perovskites is used in the reduction of temperature and ageing processes. Surface area of Alfa alumina and perovskites is similar. During the test of Nickel aluminates, combination of nickel and alumina takes place. In the preparation of provskites Ni and Al are suitable for methane reforming reactions. Nickels catalysts are finding always increasing utilization in electrochemical energy exchange systems: chemical power sources and electrolyzers. The activity in hydrogen reaction in alkaline electrolytes, the nickel and its alloys obtain the second position after platinum metals.
Pt [91], [92]. Powders of copper, nickel and cobalt are broadly used for the reason that they possess good magnetic, catalytic and electronic properties. Investigations have been concluded that when one metal was associated with another metal in trimetallic form, the properties of resultant material can improved with respect to those of hygienic metals. Lately, there is a rising concern in the preparation of Ni-based alloy nanoparticles because of its exclusive selectivity [93] and catalytic movement [94] than monometallic nickel. Catalysts which have nickel are most commonly used because of low cost, fast turnover rate and higher stability [95]. Nickel metal particles have active sites [96]. This active sites increase the carbon deposition on surface of materials [97], [98]. Nickel catalysts are in general merit analysis because they are less luxurious than rhodium or palladium catalysts, a feature that is useful for significant experiments and for industrial applications. When we dilute the nickel metal with another metal like Cu, Fe, Al, Mn etc. we can also prevent the sintering of metal particles [99], [100], [101], [102], [103]. The nickel complexes are knowledgeable for their catalytic activity with cyclotrimerization of phenylacetylene. This reaction is more important in natural product synthesis. On the other hand when temperature is close to the reforming temperature Co or Ni formed the trimetallic, perovskites (La-Ni-Fe or La-Co-Fe) which can be reduced to form active metallic species [104], [105]. Trimetallic Ni perovskites is used in the reduction of temperature and ageing processes. Surface area of Alfa alumina and perovskites is similar. During the test of Nickel aluminates, combination of nickel and alumina takes place. In the preparation of provskites Ni and Al are suitable for methane reforming reactions. Nickels catalysts are finding always increasing utilization in electrochemical energy exchange systems: chemical power sources and electrolyzers. The activity in hydrogen reaction in alkaline electrolytes, the nickel and its alloys obtain the second position after platinum metals.
2.3. Pd-based trimetallics
Palladium based alloys show the superior solubility and permeability of hydrogen as compared to pure Palladium. Particularly Ag Pd alloy is under research studies because of its high permeability for hydrogen. Pt and Pd- based core-shell colloidal nanoparticles are attractive due to their excellent tunable actions. Ag
Pd alloy is under research studies because of its high permeability for hydrogen. Pt and Pd- based core-shell colloidal nanoparticles are attractive due to their excellent tunable actions. Ag Pd nanoparticles increase selectivity, due to the presence of the sub-surface of Ag. Ag
Pd nanoparticles increase selectivity, due to the presence of the sub-surface of Ag. Ag Pd alloy have incredible selectivity for the partial hydrogenation of acetylene to ethylene. As catalyst Pd-rich surface dissociate H2and catalyze the hydrogenation reaction. However Pd is the most efficient electrode in acidic medium for ORR (Oxidation Reduction Reaction). Activity can be increased by various bimetallics and trimetallics Pd alloys such as Pd
Pd alloy have incredible selectivity for the partial hydrogenation of acetylene to ethylene. As catalyst Pd-rich surface dissociate H2and catalyze the hydrogenation reaction. However Pd is the most efficient electrode in acidic medium for ORR (Oxidation Reduction Reaction). Activity can be increased by various bimetallics and trimetallics Pd alloys such as Pd Cr, Pd
Cr, Pd Ni, Pd
Ni, Pd Co, Pd
Co, Pd Fe, Pd
Fe, Pd Cr, Pd-Co-Au and Pd-Co-Mo [106], [107], [108], [109], [110], [111], [112], [113], [114]. The observed increase in the oxidation reduction reaction activity for Pd alloys has been reported, and various theories were proposed or discovered to explain the increase activity of Pd alloys related to that of Pd [106], [107], [108], [109], [110], [111], [112], [115], [116]. The electronic property of Pd like Pt-alloys might be related to the observed increased in the ORR activity of Pd alloys. But the reason for increased ORR activity of Pd alloys is not properly clear. On the other hand in the recent work by using water/triton-X-100/propanol-2/cyclohexane micro-emulsion system carbon supported Pd and Pd-Co-Au alloys nanoparticles has been prepared. By ultraviolet photoelectron spectroscopy (UPS) the role of alloying element towards oxygen reduction activity of Pd catalyst is studied. In addition, Pt-based catalyst with elemental Pd is also extremely opposed to CO poisoning due to the many active oxygen giving groups such as PdOx, PdO and the catalytic presentation can be considerably enhanced compared with clean Pt catalyst [117], [118], [119], [120], [121].
Cr, Pd-Co-Au and Pd-Co-Mo [106], [107], [108], [109], [110], [111], [112], [113], [114]. The observed increase in the oxidation reduction reaction activity for Pd alloys has been reported, and various theories were proposed or discovered to explain the increase activity of Pd alloys related to that of Pd [106], [107], [108], [109], [110], [111], [112], [115], [116]. The electronic property of Pd like Pt-alloys might be related to the observed increased in the ORR activity of Pd alloys. But the reason for increased ORR activity of Pd alloys is not properly clear. On the other hand in the recent work by using water/triton-X-100/propanol-2/cyclohexane micro-emulsion system carbon supported Pd and Pd-Co-Au alloys nanoparticles has been prepared. By ultraviolet photoelectron spectroscopy (UPS) the role of alloying element towards oxygen reduction activity of Pd catalyst is studied. In addition, Pt-based catalyst with elemental Pd is also extremely opposed to CO poisoning due to the many active oxygen giving groups such as PdOx, PdO and the catalytic presentation can be considerably enhanced compared with clean Pt catalyst [117], [118], [119], [120], [121].
2.4. Ag-based trimetallics
These types of catalysts have high chemical purity and specific area with low bulk density. This leads to decreased utilization of catalyst for specific reaction. These types of catalyst used for the preparation of formaldehyde and varnish as a disinfectant. Ag based catalyst helps in the formation of acetaldehyde from ethyl alcohol and production of formaldehyde from methanol etc. The prior studies have also examined Ag or Ni based alloy catalysts as cathode electro catalysts because of their high ORR activity and good methanol tolerance. It is found that alloying Pt with either Ag or Ni shows the better ORR activity. Ag can significantly reduce the Gibbs free energy of the electron transfer steps in ORR for a multi component alloy catalyst. Ultimately results in the enhancement of ORR kinetics. For example the graphene supported Ag@Co-Fe NPs shows better catalytic activity than the SiO2, carbon black, and g-Al2O3 supported or support-free counterparts. The synthesized Ag@Co-Fe/graphene NPs show excellent durable stability. The Au-Pt-Ag trimetallic nanoparticles were prepared by reduction of the equivalent ions with fast insertion of NaBH4 and they show the superior catalytic activity for aerobic glucose oxidation. Pd-Ni-Ag trimetallic nanoparticles are prepared by the impregnation method. These are highly selective, active and reusable catalyst in the formic acid decomposition. Carbon supported trimetallic nanoparticles Pd-Sn-Ag/C have been prepared by sodiumborohydride (NaBH4) reduction method. The addition of third metal, including the Ag, Co and Ni can significantly increase the electro-catalytic activities of Pd-Sn/C catalyst for formic acid and ethanol electro-oxidation. The Ag-Ni/Pt-Ag-Ni NPs/C catalyst exhibits superior oxygen reduction reaction (ORR) activity and enhanced the durability compared with the commercial Pt/C catalyst. In the presence of methanol the Ag-Ni/Pt-Ag-Ni NPs/C catalyst displays much higher methanol acceptance than the commercial Pt/C catalyst in potassium hydroxide (KOH) solution. Ag-Cu-Au trimetallic clusters exhibits superior catalytic selectivity and activity, compared with the corresponding bimetallic and monometallic clusters. Trimetallic clusters have been synthesized experimentally to increase the catalytic performance of the bimetallic clusters by the addition of third metal.
3. Various methods for trimetallics synthesis
3.1. Micro-wave method
Microwave (MW) dielectric heating is fast rising as a widely accepted rapid processing technology for synthesis of variety of nanoparticles as it controls the size distribution of the nanoparticles [122]. MW heating is not only fast method but also gives extremely mono dispersed nanoparticles than conservative heating method. MW method has been also employed for preparation of trimetallics nanoparticles. Au-Pd-Pt colloidal nanocomposites have been prepared by microwave (MW) irradiation method using 160-800 W. In this method, the mixture was stirred under nitrogen atmosphere and trisodium citrate was used as a reducing agent. The preparation of trimetallic Au-Pd-Pt colloidal nanocomposites is shown in the Fig. 1. Au-Pt-Ag trimetallic nanocomposites have been also synthesized by microwave method. The ionization potentials of Pt, Au, Ag, and Pd are 8.620, 9.225, 7.576, and 8.340 eV, respectively. The detail of procedure is shown in Fig. 2. For the preparation of trimetallic nanoparticles which are of nano dimension from the aqueous solution, the poly (N-vinyl-2-pyrrolidone) (PVP) was used as a stabilizing agent for the synthesis of dissimilar combinations of various trimetallic nanoparticles.
 Fig. 1. The MW irradiation method for the preparation of trimetallic Au/Pd/Pt colloidal nanocomposites, ionization energy of the corresponding bulk metals as in set figure [123].
Fig. 1. The MW irradiation method for the preparation of trimetallic Au/Pd/Pt colloidal nanocomposites, ionization energy of the corresponding bulk metals as in set figure [123]. Fig. 2. MW irradiation method for the preparation of Au-Pt-Ag trimetallic nanoparticles [124].
Fig. 2. MW irradiation method for the preparation of Au-Pt-Ag trimetallic nanoparticles [124].3.2. Co-precipitation method
In co-precipitation method, the necessary metal cations from a general medium are co-precipitated commonly as carbonates, hydroxides, formats or citrates and oxalates etc. These precipitates are calcined at suitable temperatures to form the final powder. Fig. 3 decapitates typical co-precipitation method for nano and micro particles synthesis. Gravimetric analysis, involves the precipitation of analyte and calculation of mass to regulate its concentration. The co-precipitation method is a complicated method because undesired impurities commonly also co-precipitate with the analyte, subsequent in true additional mass. This problem can commonly alleviated by re-dissolving the sample and precipitating it another time. Inclusion occurs when the contamination inhabits a frame site in the crystal structure of the transporter which results in a crystallographic fault. This can be revealed when the ionic radius and charge of the contamination are analogous to those of the transporter. An adsorbate is a contamination that is slightly adsorbed to the outside of the precipitate. An occlusion arises when an adsorbed contamination becomes physically surrounded inside the crystal. The trimetallic nanocomposite CeO2-ZnO-ZnAl2O4 has been prepared by co-precipitation method. A novel trimetallic nanocomposite was synthesized at a temperature of around 220 °C. The band gap energy of pure CeO2 is 3.2 eV. CeO2 is measured as a candidate for replacing silicon dioxide in electronic appliances. Zinc oxide (ZnO) is a distinctive material having a wide-band gap semiconductor with the band gap energy of 3.3 eV. Another example of co-precipitation method is synthesis of Fe-Mg-La trimetallics adsorbent. A new Fe-Mg-La adsorbent with FeIII:MgII:LaIIIatratio of 2:1:1 has been synthesized by co-precipitation method. Fe-Mg-La trimetallics nanocomposite has been used as an adsorbent for adsorption of fluoride ions from drinking water. The adsorbent was amorphouswith distinct dimensions. The superior adsorption of fluoride was found at low pH while the pH of optimal solution was 4.0. The highest adsorption capacities of 185.2 and 270.3 mg/g were obtained at pH 7 and 4, respectively.
 Fig. 3. Typical co-precipitation method for micro and nano particle synthesis.
Fig. 3. Typical co-precipitation method for micro and nano particle synthesis.3.3. Hydrothermal method
This method is used for the synthesis of single crystals and relates to the solubility of precursor minerals in reaction medium at under high pressure in an autoclave. The growth of crystal depends upon the temperature gradient of reaction process. Possible advantages of the hydrothermal method over the other types of crystal growth include the ability to form crystalline phases which are not stable at the melting point. This technique is also predominantly used for the growth of huge good-quality crystals although protective switch over their configuration. A large number of compounds belonging to practically all classes have been prepared under hydrothermal conditions: elements, simple, complex oxides, tungstates, molybdates, carbonates, germinates and silicates etc. Hydrothermal synthesis is normally used to grow synthetic quartz, gems and the single crystals with commercial value. For example Ni-W-Mo-S trimetallic catalysts have been synthesized by one step hydrothermal method using ammonium thiocarbamide and heptamolybdate in water. From these catalyst,W-Mo-0.5 shows the maximum activity in the Hydrodeoxygenation (HDO) of p-cresol: the changes was high to 97.9% with a deoxygenation rate of 97.56% at 300 °C and the equivalent reaction rate constant (k) was 0.0275 mL/(mg catalyst). The HDO activity and HYD (hydrogenation-dehydration route) selectivity of Ni-Mo-W-S catalysts depends on their active sites and the proportion of corner sites. The synthesis of trimetallic Pd1NiyAl0.5composite nanoparticles is another example of hydrothermal method used for trimetallic synthesis as mentioned in above section. It is first time that the trimetallic Pd1NiyAl0.5 nanoparticle catalysts with different atomic ratios were synthesized by hydrothermal technique in the presence of metal distilled water, MWCNTs (multi-walled carbon nanotubes) and precursors. Various methods of synthesis of nanoparticles, their nanocomposites and their applications are given in the Table 1.
Table 1. Synthesis of nanoparticles, nanocomposites and their applications.
| Sr. No. | Trimetallic nanoparticles and their composites | Method of Synthesis | Applications | References |
|---|---|---|---|---|
| 1. | Au-Pt-Ag trimetallics nanocomposite | MW reduction method | Strategic green synthesis. | [125] |
| 2. | Trimetallic core shell (Pd,Co)@Pt nanoparticles | UPS reactions | Electrocatalysis | [126] |
| 3. | Trimetallic Ni-Mo-W catalysts | Co-impregnation (Ni-Mo-W) and mechanical mixing (Ni-Mo-Ni-W) | Hydro-desulfurization | [127] |
| 4. | Cobalt oxide loaded TiO2/reduced grapheme oxide nanocomposite | Sol gel method | Photo degradation of 2- chlorophenol under visible light irradiating | [128] |
| 5. | Pd-Co-Au trimetallics | Micro-emulsion method | Fuel cells applications | [129] |
| 6. | Ni-Pd-Pt nanoclusters | Hummers method | Catalyst for ethanol electro oxidation | [130] |
| 7. | Ru-Co-Ce trimetallics hybrid nano-mixed oxide | – | Allylic oxidation of cyclohexene | [131] |
| 8. | Co-Ni-Cu trimetallic alloy nanocrystals | Hydrazine reduction method | Good catalytic activity on thermal decomposition of AP and CSPs. | [132] |
| 9. | Au-Pt-Pd nanocomposite | One-pot in-situ assembly method | Electrocatalysis | [133] |
| 10. | Fe-Mg-La triple-metal composite | One-step co-precipitation method | Fluoride removal | [134] |
| 11. | Trimetallic nickel-palladium-gold hollow nanoparticles | – | Methanol electrooxidation | [135] |
| 12. | Au-Pt-Ag trimetallic nanoparticles | – | High catalytic activity for aerobic glucose oxidation | [136] |
| 13. | Trimetallic Ni-Cu-Zn | Glycine-nitrate process. | Electrochemical oxidation of hydrocarbons should | [137] |
| 14. | Trimetallic Ni-Mo-W | – | HDS | [138] |
| 15. | Trimetallic nanocomposite CeO2–ZnO-ZnAl2O4 | Co-precipitation method | For replacing silicon dioxide in electronic appliances | [139] |
| 16. | Trimetallic Pd-Ni-Ag nanoparticles | Wet-impregnation followed by simultaneous reduction method | Reusable catalyst in the formic acid decomposition | [140] |
| 17. | Trimetallic Pt-Re-Ge/Al2O3 and Pt-Ir-Ge/Al2O3 | Catalytic reduction method | Dehydrocyclization and isomerization | [141] |
| 18. | Au-Pt-Pd trimetallic | – | Catalytic activity for the glucose oxidation | [142] |
| 19. | Pt-Fe-Ni tri-metallic alloy nanoparticles | – | Electrocatalyst for oxygen reduction reaction | [143] |
| 20. | Pt-Ru-Sn/C trimetallic electrocatalysts | Pechini method | Ethanol oxidation in direct fuel cell | [144] |
| 21. |
Plurimetallic Pt Sn and Pt–Ir–Sn/Al2O3reforming catalysts Sn and Pt–Ir–Sn/Al2O3reforming catalysts |
Catalytic reduction method | Production of high-octane gasoline, aromatics and hydrogen from naphtha | [145] |
| 22. | Pt-Ni-Ru-(O2) trimetallics | – | Development of Pt-based catalyst | [146] |
| 23. | Yttrium-aluminum-iron and yttrium-cerium-iron citric complexes | Polymerized complex method | – | [147] |
| 24. | trimetallic Ni-MW/γ-Al2O3 | Co-impregnation method | Gas oil hydrotreating | [148] |
| 25. | Platinum-nickel-iridium ternary electrocatalyst | Facile approach method | Oxygen reduction reaction | [149] |
| 26. | (Ni, Co and Ag) on electrocatalytic activity improvement of Pd-Sn-based catalysts | Borohydride reduction method | Ethanol and formic acid electro-oxidation | [150] |
| 27. | Pt-Ru-Pt trimetallics | – | Promoting CO electro-oxidation | [151] |
| 28. | Ru-Mn-Cu–Al2O3 oxide catalysts | Wet impregnation method | CO2/H2 methanation in natural gas | [152] |
| 29. | La-Mo-V mixed-oxide | Sol-gel method | Direct synthesis of acetic acid | [153] |
| 30. | Ag-Ni/Pt-Ag-Ni nanoparticles | Seed-mediated growth method | As methanol-tolerant oxygen reduction electrocatalysts | [154] |
| 31 | Cu@Fe-Ni core shell nanoparticles | Facile and one-step method | Hydrolytic dehydrogenation of ammonia borane | [155] |
| 32 | Pd-Pt and Pt-Pd-Au trimetallics | NaBH4 reduction technique | Electro-oxidation of glucose in direct glucose fuel cell | [156] |
| 33. | Pt-Re-Sn trimetallics | Impregnation method | Electro-oxidation of ethanol in direct ethanol fuel cell | [157] |
| 34. | Sn-3.5Ag-XZn alloy nanoparticles | Chemical reduction method | Lead-free solder applications | [158] |
| 35. | Trimetallic NiMoW, CoMoW | Impregnation method | HDS | [159] |
| 37. | Pt-Ru/C Electro catalysts | – | Methanol and ethanol oxidation | [160] |
| 38. | Fe-Ni/Cu nanoparticles | – | Hydrous hydrazine (N2H4·H2O) decomposition | [161] |
| 39. | Pd-Ni-P nanoalloy | Facile wet chemical process | Ethanol electro-oxidation in alkaline media | [162] |
| 40. | Trimetallic Pd-Sn-Au/Al2O3 and Pd-Sn-Au/SiO2 catalysts | Catalytic process | Denitration of drinking water | [163] |
| 41. | tri-metallic Pt-Ir-Sn/C catalysts | Impregnation reduction method | Electro-oxidation of ethanol in direct ethanol fuel cell | [164] |
| 42. | Pd-Pt-Cu trimetallic | One-pot liquid-phase chemical reduction routes | Formic acid electro-oxidation | [165] |
| 43. | Pt-Cu-Au trimetallic | Facile electrochemical approach | Formic acid oxidation | [166] |
| 44. | Platinum-palladium-cobalt trimetallics | Facile one-pot solvothermal method | Ethylene glycol oxidation | [167] |
| 45. | Au/Fe/Ag hybrid nanoparticles | – | For the synthesis of α,β- and β, β-dichloroenones. | [168] |
| 46. | Pt/Ni/Cu nanoparticles | Facile wet chemical method | For hydrogen evolution reactions | [169] |
| 47. | Pt-Pd/Cu trimetallic nanostructure | Galvanic replacement method | Electrocatalytic oxidation of glycerol | [170] |
| 48. | Trimetallic AuPdPt nanoparticles | – | Detection of typical bladder cancer biomarker nuclear matrix protein 22 (NMP22) | [171] |
| 49. | Pd-Fe-Ni trimetallic nanoparticles | Otsu's method. | An elegant approach to developing a novel biotin biosensor | [172] |
| 50. | gold bimetallic and trimetallic nanoparticles | – | Selective oxidation | [173] |
| 51. | Au@PtPd nanoparticles | Facile method | Signal amplification on lab-on-paper device | [174] |
| 52. | trimetallic Au-core/AgPt-shell nanorattles and mesoporous carbon | Citrate-reduction of Frens | Detection of zearalenone | [175] |
| 53. | Pt-Ni-Au trimetallic nanoalloys | – | Highly efficient catalyst for hydrogen generation | [176] |
| 54. | Pt-based trimetallic nanoparticles | Chemical reduction method | Improved catalytic activity towards methanol oxidation | [177] |
| 55. | Trimetallic AuPdPt clusters | Monte Carlo method | Enhance catalytic activity towards methanol oxidation | [178] |
| 56. | CoAgPd nanoparticles | Facile one step method | Efficient hydrogen generation from formic acid at room temperature | [179] |
| 57. | Trimetallic Al-HMS supported catalysts | – | Hydroconversion of n-heptane | [180] |
| 58. | Iron-containing mono-, bi- and tri-metallic | – | Degradation of toxic halogenated organic compounds | [181] |
| 59. | RuCuCo nanoparticles | In-situ reduction | Hydrolysis of ammonia borane | [182] |
3.4. Selective catalytic reduction method
The term selective catalytic reduction (SCR) is used to explain the chemical reaction in which dangerous nitrogen oxides (NOX) in exhaust gas are changed into water (H2O) and nitrogen (N2). In combination with interior engine technologies, such as exhaust gas recirculation (EGR), particularly low nitrogen oxide emissions can be obtained with low fuel consumption. Urea is the most important operating fluid currently used in selective catalytic reduction (SCR) systems; alternatives to the urea agent are presently being explored. Selective Catalytic Reduction (SCR) technology is one of the most important cost-effective and fuel-efficient methods available to decrease the emissions rate. For example trimetallic Pt-I-Sn/alumina and Bimetallic Pt-Sn/alumina catalysts were synthesized by deposition of metal tin by a method called catalytic reduction method. According to catalytic reduction method, the metal tin was deposited between tin salt SnCl4 with the help of surface redox reaction. Then, the hydrogen activated on the parent catalyst and dissolved it in a hydrochloric solution. The essential analysis of tin relaxed in the solution and in the catalyst was explained by atomic absorption spectroscopy. Synthesis of Sn-3.5Ag-XZn alloy trimetallic nanoparticles is another example of reduction method. The chemical precipitation was accepted out by using poly-m-vinyl 2-pyrrolidone (PVP) as a stabilizer and NaBH4 (sodium borohydride) as a reducing agent. PVP played an important function in the chemical reduction of Sn-3.5Ag-XZn alloy trimetallic nanoparticle.
3.5. Micro-emulsion method
The micro-emulsion method can be defined as an isotropic and thermodynamically stable system, which establishes the minor droplets, distinct in immiscible solvents and amphiphilic surfactant species, on the surface of the micelle. The micro-emulsion mediated synthesis has been mainly used for the dimension selective synthesis and self gathering of nanoparticles due to the outstanding control on the particle size, which can performed through modification of micelle. For example the Pd-Co-Au trimetallics have been synthesized by micro-emulsion method. The micro-emulsion system used in this study mainly consist of propanol-2 as a co-surfactant, Triton-X-100 as a surfactant, cyclohexane as the constant oil phase, and either the Pd-Co-Au hydrazine solution as the dispersed aqueous phase. Synthesis of Pt-Ru-Co trimetallic nanoparticles is another example of micro-emulsion method. The composition of Pt-Ru-Co nanoparticles can be restricted or controlled by adjusting the primary metal salt solution and synthesis conditions. Pt-Ru-Co trimetallic nanoparticles have increased catalytic activity towards methanol oxidation as compared to Pt Ru bimetallic nanoparticles. The major features of the micro-emulsion reduction method for synthesizing mixed metal nanoparticles are the acceptance and accurateness of composition control. Thus synthesis of nanoparticles by micro-emulsion method gives a suitable control of size and composition.
Ru bimetallic nanoparticles. The major features of the micro-emulsion reduction method for synthesizing mixed metal nanoparticles are the acceptance and accurateness of composition control. Thus synthesis of nanoparticles by micro-emulsion method gives a suitable control of size and composition.
4. Various nanocomposites of trimetallics
The nanocomposites of trimetallics have been synthesized with inorganic and organic compounds such as: graphene, carbon, gelatin, starch, cellulose, alginate, chitosan, collagen, and Al2O3 etc. Lang et al., synthesize the graphene supported trimetallic Ag-Co-Fe core-shell nanoparticles for hydrolytic dehydrogenation of amine boranes. The synthesized trimetallic nanoparticles are well attached on the graphene and show the higher catalytic activity than the conventional catalysts, such as the SiO2, carbon black, and g-Al2O3 etc. The graphene has lots of advantages such as high specific surface area [183], chemical stability, thermal stability [184], superior electrical conductivity [185]and outstanding charge carrier mobility [186] etc., which could be a perfect substrate for increasing and loading metal nanoparticles (NPs). As shown in Fig. 4, GO can be measured as individual sheets of graphene decorated with O2functional groups on both the basal edges and planes [187], e.g. hydroxyl and carbonyl. The metal ions are combined with the O2 functional groups strongly and easily by the electric interaction between the negative (−) charge of O2groups and the positive (+) charge of metal ions. After that the metal ions are condensed by methylamine borane (MeAB) and dispersed consistently on the graphene. The discharge of H2 is because of the attack of H2O on a transient M-H [188] and at last obtained the metaborate and H2.
 Fig. 4. Schematic illustration (The orange hemisphere denotes GO; the grey hemisphere represents graphene). (For interpretation of the references to color in this legend [188].
Fig. 4. Schematic illustration (The orange hemisphere denotes GO; the grey hemisphere represents graphene). (For interpretation of the references to color in this legend [188].Trimetallic Ni-Pd-Pt alloy nanoclusters are consistently dispersed on DNA modified graphene sheets. In the electrochemical measurements, because of the synergistic effect of Ni, Pd and Pt, the Ni-Pd-Pt/DNA-rGO catalyst shows superior stability and greater electro-catalytic activity than bimetallic counterparts. The prepared trimetallic nanoparticles Ni1-Pd1 −-Pt1/DNA-rGO is a capable anode catalyst with low cost, high activity and stable performance for electro-oxidation of ethanol. Li Wang et al.; fabricated the graphene oxidesupported Au-Pt-Pd trimetallic nanocomposite for increasing the electro-catalytic capability. The CRGO-Au-Pt-Pd has more energetic surface area as compared to the Au-Pt-Pd nanoparticles without CRGO, which contributes to the catalytic improvement of the composite. Thus, the chemical reduced graphene oxide CRGO–Au-Pt-Pd/GCE exhibits superior performance for the electro-oxidation of ethanol, methanol and formic acid compared with Au-Pt-Pd/GCE. Tengfei Li et al.; prepared the nitrogen doped Graphene supported Pd-Ru-Bi trimetallic catalysts with synergistic effects for increasing the electro-oxidation of ethylene glycol. The durability of the catalyst is also considerably enhanced. The distinctive synergistic effects of external Bi in Pd-Ru-Bi/NG and Ru catalyst are responsible for the high electro-catalytic nature. The process of synthesis of Ru-Pd-Bi/NG nanoparticles is shown in Fig. 5.