1. Introduction
1.1. Energy storage for renewable resources
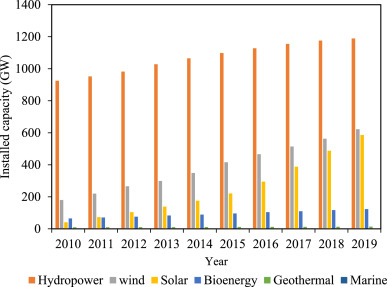
Renewable energy resouces can address the challenges faced due to conventional fuels which facilitates the formation of harmony between energy supply, ecological security, and economic viability (Fadl and Eames, 2019).This scenario drives to explore renewable energy resources, such as solar, wind, hydro-energy, biomass, tidal power, geothermal energy, etc. as potential alternatives. In this context, the increase in penetration of renewable sources in the energy market can be easily marked from Figure-1and 2 (Renewable capacity statistics, IRENA, 2016). (see Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12, Table 13, Table 14, Table 15, Table 16, Table 17, Table 18, Table 19, Table 20, Table 21, Table 22, Table 23, Fig. 1, Fig. 4, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 13, Fig. 14, Fig. 15, Fig. 16, Fig. 17, Fig. 18, Fig. 19, Fig. 20, Fig. 21, Fig. 22, Fig. 23, Fig. 24, Fig. 25, Fig. 26, Fig. 27, Fig. 28, Fig. 29, Fig. 30, Fig. 31, Fig. 32, Fig. 33, Fig. 34, Fig. 35, Fig. 36, Fig. 37, Fig. 38, Fig. 39, Fig. 40, Fig. 41, Fig. 42)
Table 1. Energy storage technologies.
| Storage technologies | Maximum power rating (MW) | Discharge time (hrs) | Lifetime (years) | Energy density (kWh/m3) | Efficiency (%) | |
|---|---|---|---|---|---|---|
| Mechanical |
Pumped Hydro |
3000 | 4–16 | 30–60 | 0.2–2 | 70–85 |
| Compressed air | 1000 | 2–30 | 20–40 | 2–6 | 40–70 | |
| Flywheel | 20 | Sec-mins | 20,000-100000 | 20–80 | 70–95 | |
| Chemical | Fuel cell | NA | NA | >1000 cycles | 2–50 | |
| Organic molecular storage | ||||||
| Electro-chemical | Li-ion battery | 100 | 1min-8h | 6–40 | 200–400 | 85–95 |
| Lead-acid battery | 100 | 1min-8h | 6–40 | 50–80 | 80–90 | |
| Thermal | Sensible | 150 | hrs | 30 | 70–210 | 80–90 |
| Latent | ||||||
| Thermochemical | ||||||
| Electrical | Capacitor | 0.01–1 | Seconds-60min | 10,000-100000 cycles | 2–10 | 90–95 |
| Supercapacitor | 0–0.3 | Miliseconds-60min | 104-106 cycles | 1–30 | 65–100 | |
| Magnetic storage | 0.1–10 | Miliseconds-8sec | 104-105 cycles | 0.2–10 | 75–99 | |
| Flow battery | 100 | hrs | 12,000-14000 | 20–70 | 60–85 | |
| Hydrogen | 100 | Mins-week | 5–30 | 600 at 200 bar | 25–45 | |
Table 2. Comparison between TES and ECS.
| TES | ECS |
|---|---|
| Low-grade thermal energy is stored in the TES system | High-grade electrical energy is stored in electrochemical system |
| Round trip efficiency is 75%–96% | Roundtrip efficiency is 75–85% |
| TES with silicon does not experience degradation due to cycling up to 20 years | Cycling operation deteriorates the performance |
| Storage materials are generally non-toxic |
Materials are toxic Example: Lead-Acid battery |
| Capital cost for long-duration storage can range $70–200/kWh | Capital cost for long-duration storage can range $200–300/kWh |
| TES can be designed to withstand extreme temperatures (very high or very low) | Electro-chemical battery cannot operate at extreme condition |
| Durability can stretch more than 15 years | Durability is 3–12 years |
| Less maintenance is required because of no self-discharge which leads to less life cycle cost | Monitoring and exchanging regularly can increase the lifecycle cost |
| TES system is most relevant for CST plant or nuclear reactor application | Electro-chemical battery is most relevant for on-grid storage of power from PV or wind plant |
Table 3. Central receiver CST plants across world.
| CRS plants | Maximum capacity (MW) | No. of heliostats | Tower height (m) | Annual energy production (GWh) | Country |
Completion Year |
|---|---|---|---|---|---|---|
| PS 10 Solara | 11 | 624 | 115 | 24 | Spain | 2006 |
| Julich solar towera | 1.5 | 2000 | 60 | NA | Germany | 2008 |
| Sierra suntowera | 5 | 24,000 | NA | NA | USA | 2009 |
| PS 20 Solara | 20 | 1255 | 165 | 44 | Spain | 2009 |
| Gemasolara | 17 | 2650 | 140 | 100 | Spain | 2011 |
| Ivanpaha | 392 | 173,500 | 139 | 650 | USA | 2013 |
| Mersina | 5 | 510 | 60 | NA | Turkey | 2013 |
| Crescent dunesb | 110 | 10,347 | 200 | 500 | USA | 2015 |
| Khi solar onea | 50 | 4120 | 180 | SA | ||
| Ashalima | 121 | 50,600 | 260 | 320 | Israel | 2018 |
| Mohammed bin Rashid solar parkc | 2863 | NA | 262 | 460 | UAE | 2020 |
| Atacamad | 110 | 10,600 | 243 | NA | Chile | 2021 |
- a
-
-Operational.
- b
-
- The Crescent Dunes site has not produced power since April 2019. However, Tonopah Solar Energy has stated to restart of the Crescent Dunes plant by the end of 2020.
- c
-
- This is a hybrid PV/solar thermal plant aims to be completed in 5 phases. The third phase is expected to be completed in 2020. The completed first two phases have 213 MW capacity.
- d
-
Under construction. To be completed in 2021.
Table 4. Current status of CRS and PTC technology.
| Parameters | Values | |
|---|---|---|
| CRS | PTC | |
| Operating temperature (oC) | 300–1000 | 20–400 |
| Solar concentration ratio | 150–1500 | 15–45 |
| Storage integration possibility | Highly possible with low storage cost | possible |
| Plant peak efficiency (%) | 23–35 | 14–20 |
| Grid stability | High (large TES) | Medium to high (TES) |
| Annual capacity factor (%) | 55 (with 10h TES) | 29-43 (with 7h TES) |
| Installation cost | Relatively high | Relatively low |
| Thermodynamic efficiency | High due to high operating temperature | Low due to low operating temperature |
| Maturity of technology | Pilot plants, commercial projects | Commercially proven |
| Outlook for improvement | Very significant | Limited |
| Relative rise in efficiency after improvement | 40–65 | 20 |
| LCoE ($/kWh) | 0.2–0.29 (with 6–7.5h TES) | 0.26–0.37 (no TES) |
| 0.17–0.24 (12–15h TES) | 0.22–0.34 (TES) | |
| Capital cost ($/kW) | 6400-10700 with TES | 6400-10700 with TES |
Table 5. Comparison between 2nd and 3rd Gen CST plant.
| Generation | 2nd Gen | 3rd Gen |
|---|---|---|
| Receiver outlet temperature (oC) | ~500–565 | >700 |
| CST technology | PTC, CRS, LFR | CRS |
| Heat transfer carrier | Steam or salt | Salt, particle, gas |
| Thermal energy storage | Recent design includes | Implicit |
| Power cycle | Steam Rankine cycle | Brayton cycle |
| Design cycle efficiency (%) | 38–44 | Expected to be > 50 |
| Annual solar-electric efficiency (%) | 10–20 | 25–30 |
Table 6. Merits and limitations of different integration concepts.
| Two-tank direct concept | Two-tank indirect concept | Passive concept with PCM | |
|---|---|---|---|
| Merits |
|
|
|
| Demerits |
|
|
|
Table 7. Comparison of different thermal storage technologies.
| SHS | LHS | TCES | |
|---|---|---|---|
| Mechanism | Temperature gradient | Isothermal phase transition | Reversible chemical reactions |
| Volumetric Density | Small ~50 kWh/m3 | Medium~100 kWh/m3 | High (500 kWh/m3) |
| Gravimetric density | Small~0.02–0.03 kWh/kg | Medium~0.05–0.1 kWh/kg | High (0.5-1 kWh/kg) |
| Storage period | Limited due to thermal losses | Limited due to thermal losses | Theoretically unlimited |
| Storage Temperature | charging Temperature (More than TCES) | charging Temperature (More than TCES) | Surrounding temperature (Less than SHS & LHS) |
| Maturity | Industrial-scale | Pilot Scale | Laboratory stage |
| Technology | Simple | Medium | Complex |
| Feedback | Large experimental and commercial feedback | Less experimental and no commercial feedback | No feedback |
| Flexibility | Less time to switch between charging and discharging | Less time to switch between charging and discharging | Switch between charge and discharge takes medium time |
Table 8. Correlation between properties of PCM and performance of high-temperature LHS.
| Properties of high-temperature PCM | Performance of LHS system |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 9. Qualitative value of an ideal PCM for high-temperature LHS.
| Properties | Desirable value | Results | |
|---|---|---|---|
| Physical | Density | High | Leads to a small size of storage container |
| Vapor pressure | Low | To avoid the complex design of containment | |
| Volume change | Small | Reduce the containment size | |
| Phase equilibrium | Favorable | Help towards setting energy storage | |
| Thermal | Phase change temperature | Should match the operating temperature of applications | High operating temperature leads to better efficiency for power generation. |
| Energy density | Should be as high as possible | Reduction of weight per unit volume of the storage system | |
| Thermal conductivity | Should be high | To facilitate high rate of charging and discharging | |
| Thermal stability | Should be high | Thermally stable material prevents early replacement of storage media leads to a reduction in maintenance costs | |
| Kinetic | Subcooling | Should be less than five degrees | To avoid difficulty in heat extraction from storage |
| Crystallization rate | Sufficient | Helps to reduce the duration of the cycles | |
| Chemical |
Toxicity, flammability Corrosion |
PCMs should be non-toxic, non-flammable and non-explosive | Expedites handling and operation of the plant, and, finally, safe transfer |
| Chemically stable | No reaction with PCM containment | Reduction of disposal time of material | |
| Economical | Cost | Low | Addition of financial benefit |
| Availability | Plentily available | Ensure a steady supply | |
| Environmental | CO2 footprint | Should be very low | Reduction in pollution |
Table 10. Softwares/Online databases for selection of storage medium.
| Sl No. | Tool name | Database/Software tool | Description | Reference |
|---|---|---|---|---|
| 1 |
CES Selector |
Material selection software | Mechanical, thermal, physical, optical properties of different materials are compared. Environmental impact and cost analysis are also analyzed. | (“CES selector,” 1994) |
| 2 | GRANTA MI | Material database management software | Online tool for material management and also serves as a support to integrate with modeling/simulation software. | (“GRANTA MI,” 1994) |
| 3 | IDEMAT | Online material selection database and software | Life cycle analysis for free of cost, sustainability, ecological impact assessment for more than 1000 materials. | (“IDEMAT-Industrial Design & Engineering MATerials database,“) |
| 4 | MATERIA | Online directory | Technical specifications and manufacturer details of more than 2600 new materials available without any charge | (“Materia,” 1998) |
| 5 | MatWeb | Online bibliography | Freely available content comprising specification sheet and supplier details of nearly 125,000 materials. It can be utilized as a material database in commercial software such as ANSYS Workbench and COMSOL Multiphysics. | (“MatWeb,” 1999) |
| 6 | PCMexpress | Modeling tool | Explains the effect of PCM for thermal management of the building and the economic analysis. | (“PCMexpress-A planning and simulation programme for thermal management of buildings using PCMs,” 2008) |
| 7 | ThermoCalc | Thermodynamic properties calculation software | Paid tool determines the thermophysical properties of eutectic mixtures using the built-in database of alloys. | (Andersson et al., 2002) |
| 8 |
Worksheet database with user-defined properties. |
Computational Tool | It predicts the composition, thermal & physical properties of eutectic organic PCMs. Provision of expansion of database with user-defined functions are added advantage | (Kahwaji and White, 2018). |
Table 11. Merits and limitations of organic and inorganic PCM.
| Organic PCM | Inorganic PCM | ||
|---|---|---|---|
| Merits | Limitations | Merits | Limitations |
| Solidification takes place without high degree of subcooling | Thermal conductivity is low in solid phase. | Higher thermal conductivity than organic | High degree of subcooling |
| Ability to be incorporated directly | Low heat of phase transformation | Enthalpy change is more during phase transition | Lack of thermal stability |
| Low vapor pressure during phase transition | Volumetric storage density is low | Lower volumetric expansion | Phase segregation |
| Self-nucleating properties | Low heat capacity | High volumetric energy density | Few have more weight |
| Ability to melt congruently | High volumetric expansion | Large heat storage capacity | Few inorganic PCMs show high volumetric change |
| Compatible with conventional containment material | Low density | Sharp phase-change | Not suitable for a few building materials |
| No segregation | Lower operating temperature range than inorganic PCM | Operating temperature spreads over a wide range of temperature to suit high-temperature storage | Incongruent melting and dehydration during thermal cycling |
| Chemically stable | Flammable | Less costly | Chemical instability |
| Safe, non-reactive, and recyclable | Require large surface area | Non-flammable, recyclable | Corrosive & Prone to degradation |
| Organic PCMs have their transition temperature close to human thermal comfort range between 18 °C and 30 °C. | Organic PCMs decompose at higher temperatures | Inorganic PCMs can operate at higher temperature | Inorganic PCMs do not suit for thermal comfort application (Except salt hydrates) |
Table 12. Properties of high-temperature inorganic salts and their mixtures.
| Single salt | Melting point (°C) | Heat of fusion (J/g) | Density (kg/m3) |
Storage Capacity (MJ/m3) |
Cost ($/tonne) | Mixture of Salts |
Melting Point (°C) |
Latent Heat (J/g) |
|---|---|---|---|---|---|---|---|---|
| LiF | 849 | 1041 |
2640 (S) 1810 (L) |
2756.2 | 32,500 | KF (55%)/KCl (45%) | 605 | 407 |
| NaF | 996 | 794 |
2558 (S) 1948 (L) |
2031.1 | 1610 | LiF (60%)/NaF (40%) | 652 | 816 |
| KF | 858 | 507 |
2370 (S) 1910 (L) |
1109.2 | 5300 | NaF (65%)/CaF2 (23%)/MgF2 (12%) | 743 | 568 |
| MgF2 | 1263 | 938 | NA | NA | 870 | NaF (64%)/MgF2 (20%)/KF (16%) | 804 | 650 |
| CaF2 | 1418 | 381 |
3180 (S) 1910 (L) |
1243.4 | 120 | NaF (75%)/MgF2 (25%) | 832 | 627 |
| LiCl | 610 | 416 |
2070 (S) 1502 (L) |
912.9 | 8000 | KCl(21%)/NaF(17%)K2CO3(62%) | 520 | 274 |
| NaCl | 801 | 482 | 2160 (S) | 907.2 | 49 | KCl(40%)/KF (23%)/K2CO3(37%) | 528 | 283 |
| KCl | 771 | 353 |
1980 (S) 1527 (L) |
698.9 | 455 | KCl (45%)/KF (55%) | 605 | 407 |
| MgCl2 | 714 | 454 |
2320 (S) 1680 (L) |
1048.6 | 342 | K2CO3 (50%)/Na2CO3 (50%) | 710 | 163 |
| CaCl2 | 772 | 253 |
2150 (S) 2085 (L) |
544 | 200 | NaF (75%)/MgF2 (50%) | 832 | 650 |
| Li2CO3 | 732 | 509 | 2110 (S) | 1074 | 7050 | LiF (67%)/MgF2 (33%) | 746 | 947 |
| Na2CO3 | 858 | 165 |
2533 (S) 1972 (L) |
699.1 | 324 | NaF(65%)/CaF2(23%)/MgF2(12%) | 745 | 574 |
| K2CO3 | 900 | 202 | 2290 (S) | 540.4 | 1100 | LiF(33.4%)/NaF2(49.9%)/MgF2(17.1%) | 650 | 860 |
| Mg2CO3 | 990 | 698 | NA | NA | NaCl (38.5%)/NaBr (23%)/Na2MoO4(38.5%) | 612 | 168 | |
| CaCO3 | 1330 | NA | 2930 (S) | 416.1 | NA | CaCl2(38.5%)/CaSO4(11%)/CaMoO4(4%) | 673 | 224 |
Table 13. Properties of high-temperature metals and cost.
| Materials | Melting Point (°C) | Latent Heat (MJ/kg) | Density (kg/m3) | Specific heat (J/kg K) | Thermal Conductivity (W/m K) | Cost ($/lb) |
|---|---|---|---|---|---|---|
| Copper (Cu) | 1356 | 0.193 | 8800 | 385 | 350 | 3–3.5 |
| Nickel (Ni) | 1728 | 0.3 | 8908 | 440 | 83 | 6–6.5 |
| Chromium (Cr) | 2180 | 0.4 | 7140 | 450 | 48 | 5–5.5 |
| Vanadium (V) | 2183 | 0.45 | 6110 | 490 | 51 | 200–210 |
| Silicon (Si) | 1410 | 1.79 | 2570 | 1040 | 20 | 1.2–1.5 |
| Boron (B) | 2350 | 4.6 | 2340 | 1020 | 10 | 20–25 |
| Aluminium (Al) | 660 | 0.397 | 2800 | 900 | 204 | 1.5–2 |
| Magnesium (Mg) | 661 | 0.388 | 1746 | 1270 | 156 | NA |
| Zinc (Zn) | 419 | 0.146 | 7140 | 0.48 | 112.2 | NA |
Table 14. Research progess in inorganic salts based PCM for high-temperature LHS.
| Reference | Key inference |
|---|---|
|
Kenisarin, 2010 |
State of art high-temperature PCMs for thermal storage in the temperature range of 120–1000 °C are discussed |
| Ren et al. (2011) | 36 kinds of mixed carbonate molten salts are prepared by mixing potassium carbonate, lithium carbonate, sodium carbonate having melting point close to 400 °C |
| Olivares (2012) | Thermal stability of current generation Nitrate based salts (solar salt and HITEC) are performed and found that salts decompose above 600 °C |
| Myers and Goswami (2016) | Alkali metal derived ternary eutectic chloride salt (Nacl-CalCl2-MgCl2) is prepared for thermal storage above 500 °C in a CST system |
| Dadollahi and Mehrpooya (2017) | Alkali metal (Sodium, potassium,lithium) based chloride and fluoride salts are tested as high-temperature PCM for three different storage configurations |
| Mohan et al. (2018) | Four ternary chloride mixtures with different cation combinations (Na, K, Li, Mg) were designed using the FactSage® software. Thermal properties and thermal stability of the salts are measured |
| Mohan et al. (2019) | This paper critically discuses energy storage in fluids having thermal stability over 600 °C. The key focus is fluorides, carbonates and chloride salts |
| Vidal and Klammer (2019) | For 3rd Gen CST system, nine diffrent mixture of chlorides (Mg,K and Na) are tested with DSC and TGA and lowest melting temperature is found for MgCl2(44.7),KCl (25.8) and NaCl (29.4) (mol%) |
| Wang et al. (2020) | A mixture of different salts (NaCl–NaF–KCl) is developed by pandat software and experiment. The melting point and fusion enthalpy were found to be 604.1 °C and 398.4J/g. |
| Ding and Bauer (2021) | This paper discusses recent progress in the selection/optimization of chloride salts, determination of molten chloride salt properties, and corrosion control of construction materials (e.g., alloys) in molten chlorides |
Table 15. Research progress in metallic PCM for high-temperature LHS.
| Reference | Key inference |
|---|---|
| Riechman and Birchenall (1980) | They were the first to analyze the metals as suitable high-temperature PCM. Various alloys of Al,Cu,Mg and Si are tested and verified that Al and Si alloys have best storage densities per mass or volume |
| Wang et al. (2006) | They developed a novel high-temperature phase cahnge storage system using AlSi12 having high heat of fusion (560 kJ/kg) and thermal conductivity (160 W/mK). |
| Rodríguez-Aseguinolaza et al. (2014) | The eutectic Mg49–Zn51 alloy was identified as suitable PCM for LHS. Both solid-solid and solid-liquid phase transitions were characterized |
| Kotzé et al. (2013) | A concept was developed to integrate LHS with AlSi12 as PCM with steam generator. The analysis indicated that the cost of the AlSi12 storage material is 14.7 US$ per kWh of thermal energy storage. |
| Wang et al. (2015) | Four binary and 2 ternary alloys of Aluminum and Silicon were investigated for potential high-temperature storage |
| Wei et al. (2016) | The aluminium alloy samples are preapred and thermophysical properties are measured using DSC, laser flash apparatus. They observed that adding Cu, Zn, and Si to an aluminum alloy reduces the melting point of the alloy. |
| Fang et al. (2016) | This study characterized Mg–36%Bi, Mg–54%Bi and Mg–60%Bi (wt. %) alloys as phase change materials for thermal energy storage at high temperature and established Mg based alloys have hgih corrosion resistance than Aluminium based alloys |
| Polkowski et al. (2018) | Silicon and Boron alloys were fabricated as high-temperature PCM and their high temperature interaction with refractories was examined |
| Zeneli et al. (2019) | Numerical investigation of silicon based latent storage system was performed considering volumetric change during phase change and dendritic formations.Silicon melting was reduced with increase in Stefan number |
| Safarian and Tangstad (2020) | The properties of pure metals and alloys and specifically Si-based alloys are proposed as suitable PCM |
Table 16. Comparison between DSC and DTA technique.
| DSC | DTA |
|---|---|
|
|
|
|
|
|
|
|
Table 17. Comparison between steady and transient method for thermal conductivity measurement.
| Steady method | Transient method |
|---|---|
| The governing equation for a steady method is 1-D steady-state Fourier law | The working principle is established from the unsteady heat conduction equation |
| Conductivity measurement using steady-state includes guarded hot plate (GHP) technique | Transient thermal conductivity measurement techniques include Transient hot-strip (THS), laser flash analysis (LFA), transient plane source (TPS), and transient hot-wire (THW) |
| Because of steady behavior, this method makes the signal analysis straightforward | Transient behavior makes the signal analysis complex due to temporal variation |
| This method requires a long duration of time to attain a steady-state in actual experiments | This method provides quick and highly accurate results. |
| This is a more accepted and appropriate method to measure relatively low thermal conductivity samples | The laser flash method can measure samples with high thermal conductivity. |
Table 18. Thermophyscial properties of htfs.
| Htf | MP (°C) | Thermal Stability limit (°C) | Viscosity (Pa.s) |
Thermal Conductivity (W/m. K) |
Specific heat (kJ/kg.K) | Cost ($/kg) | |
|---|---|---|---|---|---|---|---|
| Air | – | – |
0.00003@ 600 °C |
°0.06@600°; C |
1.12 @600 °C |
0 | |
| Water/Steam | 0 |
0.00133@ 600 °C |
°0.08@600°; C | °2.42@600°; C | ~0 | ||
| Thermal oil | −20 | 300–400 | °0.001@300°; C | ~0.1 | °2.436@300°; C | 0.3–5 | |
| Organics (Biphenyl/Diphenyl oxide) | 12 | 393 | °0.0006@300°; C | ~0.01 | °1.93@300°; C | 100 | |
| Molten salts | Nitrate and nitride | ~65–220 | 500-600 | 0.003–0.03 | <0.5 | 1–1.5 | 0.5–1.1 |
| Fluorides and carbonates | ~400 | 800–900 | ~0.004@800 °C | ~1.2 | 1.2–1.3 | 1.2–1.3 | |
| Chlorides | 200–650 | 850 | °0.004@600°; C | °0.325@300°; C | 0.81 | 1 | |
| Liquid metals | Na | 98 | 883 | °0.0002@600°; C | 46@600 °C | °1.25@600°; C | 2 |
| Na–K | −12 | 785 | °0.00018@600°; C | °26.2@600°; C | °0.87@600°; C | 2 | |
| Pb–Bi | 125 | 1533 | °0.00108@600°; C | 12.8@6000C | 0.15@6000C | 13 | |
Table 19. Merits and limitations of htf.
| htf | Merits | Limitations |
|---|---|---|
| Thermal oil |
|
|
| Molten Salt |
|
|
| Direct steam |
|
|
| sCO2 |
|
|
Table 20. Comparison between fixed and variable domain method.
| Fixed domain method | Variable domain method |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 21. Charging, discharge and overall exergy efficiency of LHS.
| Efficiency | Expressions | Description |
|---|---|---|
| Charging (Φcharge) | ψstored/ψhtf | Exergy efficiency during charging is the ratio between exergy stored to total exergy entered to TES during charging |
| ψstored/ψhtf | Rate of change Exergy efficiency | |
| ψstored/ψhtf + Pump work | Pump work is also included | |
| ψstored/ψinit | Maximum possible exergy stored | |
| Discharging (Φdischarge) | ψhtf/ψpcm, init | Discharge efficiency is ratio between exergy retrieved to total exergy stored in the TES system |
| ψhtf/ψpcm | Presents the maximum possible exergy retrieved | |
| Overall (Φoverall) | ψrecovered/ψsupplied | Total exergy efficiency for the complete cycle |
| Φoverall = Φcharge × Φdischarge | Product of charging and discharging efficiency |
Table 22. Layouts of sCO2 Brayton cycle.
| Single flow layout | Split flow layout |
|---|---|
| Recuperation | Recompression |
| Intercooling | Modified recompression |
| Reheating | Preheating |
| Inter-recuperation | Turbine split flow-1 |
| Pre-compression | Turbine split flow-2 |
| Split-expansion | Turbine split flow-3 |
Table 23. Novel high-temperature TIM.
 Fig. 1
Fig. 1 Fig. 2
Fig. 2