In recent decades, a concerning rise in metabolic illnesses -- such as cardiovascular disease, high blood pressure and diabetes -- has focused scientific attention on the biology and chemistry of fat, resulting in a wealth of information about how fat cells work.
But fat cells and their metabolic activities are only part of the story.
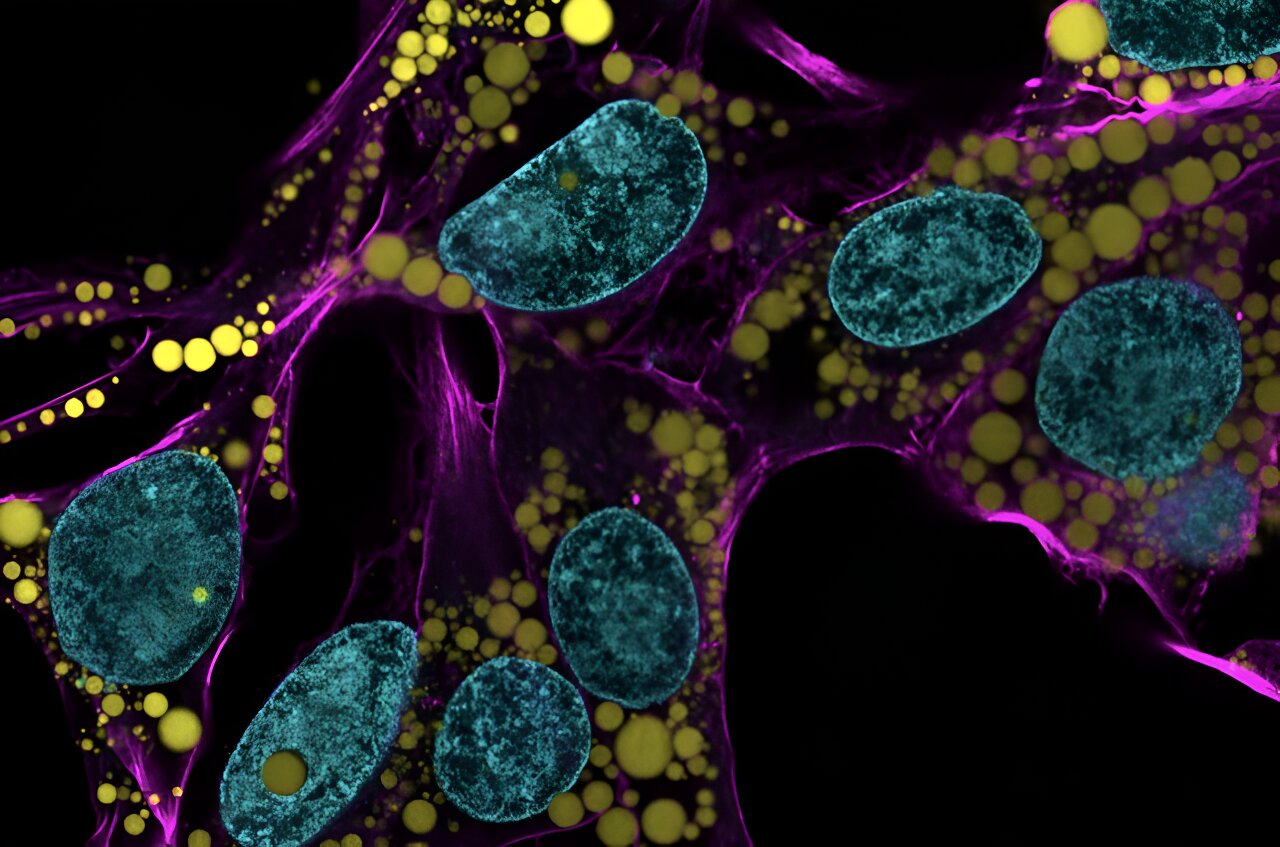
Fat-filled lipid droplets, tiny spheres of fat many times smaller than fat cells, are a growing subject of scientific interest.
Found inside many different cell types, these lipid particles have long been little understood.
Studies have begun to illuminate these droplets' participation in metabolic functions and cellular protection, but we still know next to nothing about the physical nature of fat.
Now, researchers at the University of Pennsylvania School of Engineering and Applied Science have looked beyond biochemistry to publish groundbreaking work on the physics of these droplets, revealing them to be a potential threat to a cell's nucleus.
In the August issue of the Journal of Cell Biology, they are the first to discover fat-filled lipid droplets' surprising capability to indent and puncture the nucleus, the organelle which contains and regulates a cell's DNA.
The stakes of their findings are high: a ruptured nucleus can lead to elevated DNA damage that is characteristic of many diseases, including cancer.
The study was led by Dennis E. Discher, Robert D. Bent Professor in the Department of Chemical and Biomolecular Engineering, Irena Ivanovska, Ph.D. Research Associate in Penn's Molecular and Cell Biophysics Lab, and Michael Tobin, Ph.D. Candidate in the Department of Bioengineering.
"Intuitively, people think of fat as soft," says Discher. "And on a cellular level it is. But at this small size of droplet -- measuring just a few microns rather than the hundreds of microns of a mature fat cell -- it stops being soft. Its shape has a much higher curvature, bending other objects very sharply. This changes its physics in the cell. It can deform. It can damage. It can rupture."
"Imagine," adds Ivanovska, "trying to pop a balloon with your fist. Impossible. You can deform the balloon, but you won't puncture it. Now imagine trying to pop it with a pen. That's the difference between a fat cell and a cell with small fat droplets in the body. It's a fundamental physical difference, not a metabolic one."
The team's research reframes scientific inquiry into fat, underlining that fat's role in the body is much more than just a number on the scales.
"This isn't fat canonically conceived," says Tobin. "This is about how fat works at scales smaller than a cell and poses physical risks to cellular components, even at the level of DNA."
The team's work builds on a decade of foundational research, including leading contributions by Ivanovska, into the behaviors of nuclear proteins that give the nucleus its protective structural qualities.
These proteins are dynamic, shifting levels to respond to their mechanical environments and provide what the nucleus needs to maintain its integrity.
"There's a constant process of repair to DNA damage that goes on in cells," says Ivanovska.
"For this to happen, the nucleus needs to have enough DNA repair proteins. If a nucleus is ruptured, these proteins scatter and cannot repair damage in a timely manner. This causes DNA damage accumulation and can potentially result in a cancer cell."
A cell lives in a dynamic physical and mechanical environment where things can and do go wrong.
But it also has an army of molecular helpers always working to maintain and repair it.
"The problem is," says Discher, "when a nucleus is compromised -- by toxins, overexposure to UV rays, or these fat-filled lipid droplets. Then there is a strong potential for DNA damage and that comes with consequences for health."